Analysis of the effect of HDAC inhibitors on the formation of the HIV reservoir
- PMID: 39136440
- PMCID: PMC11389399
- DOI: 10.1128/mbio.01632-24
Analysis of the effect of HDAC inhibitors on the formation of the HIV reservoir
Abstract
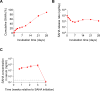
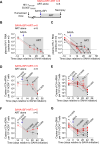
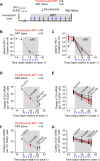
The HIV reservoir is more dynamic than previously thought with around 70% of the latent reservoir originating from viruses circulating within 1 year of the initiation of antiretroviral therapy (ART). In an ex vivo model system of HIV latency, it was reported that early exposure to class I histone deacetylase (HDAC) inhibitors might prevent these more recently infected cells from entering a state of stable viral latency. This finding raises the possibility that co-administration of HDAC inhibitors at the time of ART initiation may prevent the establishment of much of the HIV reservoir. Here, we tested the effects of the HDAC inhibitors suberoylanilide hydroxamic acid (SAHA) and panobinostat co-administered at the time of ART initiation on the formation of the viral reservoir in HIV-infected humanized mice. As previously shown, SAHA and panobinostat were well tolerated in humanized mice. Unexpectedly, co-administration of SAHA resulted in an increase in the frequency of CD4+ cells carrying HIV DNA but did not alter the frequency of cell-associated HIV RNA in HIV-infected, ART-treated humanized mice. Co-administration of panobinostat did not alter levels of cell-associated HIV DNA or RNA. Our in vivo findings indicate that co-administration of HDAC inhibitors initiated at the same time of ART treatment does not prevent recently infected cells from entering latency.IMPORTANCECurrent antiretroviral therapy (ART) does not eradicate cells harboring replication-competent HIV reservoir. Withdrawal of ART inevitably results in a rapid viremia rebound. The HIV reservoir is more dynamic than previously thought. Early exposure to class I histone deacetylase (HDAC) inhibitors inhibit these more recently infected cells from entering a state of stable viral latency in an ex vivo model of latency, raising the possibility that co-administration of HDAC inhibitors at the time of ART initiation may reduce much of the HIV reservoir. Here, we tested the effects of the HDAC inhibitors suberoylanilide hydroxamic acid or panobinostat during ART initiation on the formation of the viral reservoir in HIV-infected humanized mice. Our in vivo study indicates that in contrast to in vitro observations, the co-administration of HDAC inhibitors at the same time of ART initiation does not prevent recently infected cells from entering latency.
Keywords: histone deacetylase inhibitors; human immunodeficiency virus; latency; reservoir.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection.Retrovirology. 2016 May 21;13(1):36. doi: 10.1186/s12977-016-0268-7. Retrovirology. 2016. PMID: 27206407 Free PMC article.
-
The histone deacetylase inhibitor vorinostat (SAHA) increases the susceptibility of uninfected CD4+ T cells to HIV by increasing the kinetics and efficiency of postentry viral events.J Virol. 2014 Sep;88(18):10803-12. doi: 10.1128/JVI.00320-14. Epub 2014 Jul 9. J Virol. 2014. PMID: 25008921 Free PMC article.
-
Short Communication: The Broad-Spectrum Histone Deacetylase Inhibitors Vorinostat and Panobinostat Activate Latent HIV in CD4(+) T Cells In Part Through Phosphorylation of the T-Loop of the CDK9 Subunit of P-TEFb.AIDS Res Hum Retroviruses. 2016 Feb;32(2):169-73. doi: 10.1089/AID.2015.0347. AIDS Res Hum Retroviruses. 2016. PMID: 26727990 Free PMC article.
-
CXCR4 Targeting Nanoplatform for Transcriptional Activation of Latent HIV-1 Infected T Cells.ACS Appl Bio Mater. 2024 Aug 19;7(8):4831-4842. doi: 10.1021/acsabm.3c00456. Epub 2023 Aug 16. ACS Appl Bio Mater. 2024. PMID: 37586084 Review.
-
Histone deacetylase inhibitors for purging HIV-1 from the latent reservoir.Mol Med. 2011 May-Jun;17(5-6):466-72. doi: 10.2119/molmed.2011.00076. Epub 2011 Mar 15. Mol Med. 2011. PMID: 21424110 Free PMC article. Review.
Cited by
-
Interventions during Early Infection: Opening a Window for an HIV Cure?Viruses. 2024 Oct 9;16(10):1588. doi: 10.3390/v16101588. Viruses. 2024. PMID: 39459922 Free PMC article. Review.
References
-
- Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. 2012. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature 487:482–485. doi:10.1038/nature11286 - DOI - PMC - PubMed
-
- Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, et al. . 2014. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog 10:e1004473. doi:10.1371/journal.ppat.1004473 - DOI - PMC - PubMed
-
- Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Østergaard L, Søgaard OS. 2014. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV 1:e13–e21. doi:10.1016/S2352-3018(14)70014-1 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
