Lipases are differentially regulated by hormones to maintain free fatty acid homeostasis for insect brain development
- PMID: 39135168
- PMCID: PMC11321213
- DOI: 10.1186/s12915-024-01973-3
Lipases are differentially regulated by hormones to maintain free fatty acid homeostasis for insect brain development
Abstract
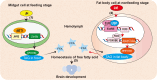
Background: Free fatty acids (FFAs) play vital roles as energy sources and substrates in organisms; however, the molecular mechanism regulating the homeostasis of FFA levels in various circumstances, such as feeding and nonfeeding stages, is not fully clarified. Holometabolous insects digest dietary triglycerides (TAGs) during larval feeding stages and degrade stored TAGs in the fat body during metamorphosis after feeding cessation, which presents a suitable model for this study.
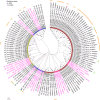
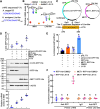
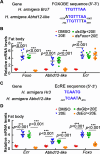
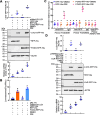
Results: This study reported that two lipases are differentially regulated by hormones to maintain the homeostasis of FFA levels during the feeding and nonfeeding stages using the lepidopteran insect cotton bollworm Helicoverpa armigera as a model. Lipase member H-A-like (Lha-like), related to human pancreatic lipase (PTL), was abundantly expressed in the midgut during the feeding stage, while the monoacylglycerol lipase ABHD12-like (Abhd12-like), related to human monoacylglycerol lipase (MGL), was abundantly expressed in the fat body during the nonfeeding stage. Lha-like was upregulated by juvenile hormone (JH) via the JH intracellular receptor methoprene-tolerant 1 (MET1), and Abhd12-like was upregulated by 20-hydroxyecdysone (20E) via forkhead box O (FOXO) transcription factor. Knockdown of Lha-like decreased FFA levels in the hemolymph and reduced TAG levels in the fat body. Moreover, lipid droplets (LDs) were small, the brain morphology was abnormal, the size of the brain was small, and the larvae showed the phenotype of delayed pupation, small pupae, and delayed tissue remodeling. Knockdown of Abhd12-like decreased FFA levels in the hemolymph; however, TAG levels increased in the fat body, and LDs remained large. The development of the brain was arrested at the larval stage, and the larvae showed a delayed pupation phenotype and delayed tissue remodeling.
Conclusions: The differential regulation of lipases expression by different hormones determines FFAs homeostasis and different TAG levels in the fat body during the feeding larval growth and nonfeeding stages of metamorphosis in the insect. The homeostasis of FFAs supports insect growth, brain development, and metamorphosis.
Keywords: 20-Hydroxyecdysone; Forkhead box O; Juvenile hormone; Lipase; Methoprene-tolerant 1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Methoprene-tolerant 1 regulates gene transcription to maintain insect larval status.J Mol Endocrinol. 2014 Aug;53(1):93-104. doi: 10.1530/JME-14-0019. Epub 2014 May 28. J Mol Endocrinol. 2014. PMID: 24872508
-
Vrille is required for larval moulting and metamorphosis of Helicoverpa armigera (Lepidoptera: Noctuidae).Insect Mol Biol. 2019 Jun;28(3):355-371. doi: 10.1111/imb.12557. Epub 2019 Jan 10. Insect Mol Biol. 2019. PMID: 30485565
-
Insulin and 20-hydroxyecdysone oppose each other in the regulation of phosphoinositide-dependent kinase-1 expression during insect pupation.J Biol Chem. 2018 Nov 30;293(48):18613-18623. doi: 10.1074/jbc.RA118.004891. Epub 2018 Oct 10. J Biol Chem. 2018. PMID: 30305395 Free PMC article.
-
Krüppel-like factor 15 integrated autophagy and gluconeogenesis to maintain glucose homeostasis under 20-hydroxyecdysone regulation.PLoS Genet. 2022 Jun 13;18(6):e1010229. doi: 10.1371/journal.pgen.1010229. eCollection 2022 Jun. PLoS Genet. 2022. PMID: 35696369 Free PMC article.
-
The genetic determination of alternate stages in polyphenic insects.Evol Dev. 2024 Sep;26(5):e12485. doi: 10.1111/ede.12485. Epub 2024 Jun 12. Evol Dev. 2024. PMID: 38867484 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

