Trop2-targeted therapy in breast cancer
- PMID: 39135109
- PMCID: PMC11321197
- DOI: 10.1186/s40364-024-00633-6
Trop2-targeted therapy in breast cancer
Abstract
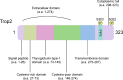
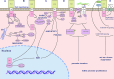
Human trophoblastic cell surface antigen 2 (Trop2) is a glycoprotein, a cellular marker of trophoblastic and stem cells, and a calcium signaling transducer involved in several signaling pathways, leading to the proliferation, invasion, and metastasis of tumors. It is expressed at a low level in normal epithelial cells, but at a high level in many tumors, making it an ideal target for cancer therapy. According to previous literature, Trop2 is broadly expressed in all breast cancer subtypes, especially in triple negative breast cancer (TNBC). Several clinical trials have demonstrated the effectiveness of Trop2-targeted therapy in breast cancer. Sacituzumab govitecan (SG) is a Trop2-targeted antibody-drug conjugate (ADC) that has been approved for the treatment of metastatic TNBC and hormone receptor-positive (HR+) and human epidermal growth factor receptor 2-negative (HER2-) breast cancer. This article reviews the structure and function of Trop2, several major Trop2-targeted ADCs, other appealing novel Trop2-targeted agents and relevant clinical trials to provide a landscape of how Trop2-targeted treatments will develop in the future.
Keywords: Antibody-drug conjugate; Breast cancer; SG; TNBC; Targeted therapy; Trop2.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Shedding light on triple-negative breast cancer with Trop2-targeted antibody-drug conjugates.Am J Cancer Res. 2022 Apr 15;12(4):1671-1685. eCollection 2022. Am J Cancer Res. 2022. PMID: 35530278 Free PMC article. Review.
-
Advances in Trop2-targeted therapy: Novel agents and opportunities beyond breast cancer.Pharmacol Ther. 2022 Nov;239:108296. doi: 10.1016/j.pharmthera.2022.108296. Epub 2022 Oct 5. Pharmacol Ther. 2022. PMID: 36208791 Review.
-
Tackling metastatic triple-negative breast cancer with sacituzumab govitecan.Expert Rev Anticancer Ther. 2021 Dec;21(12):1303-1311. doi: 10.1080/14737140.2021.1993065. Epub 2021 Oct 22. Expert Rev Anticancer Ther. 2021. PMID: 34651524
-
Trophoblast Cell Surface Antigen 2 (TROP2) as a Predictive Bio-Marker for the Therapeutic Efficacy of Sacituzumab Govitecan in Adenocarcinoma of the Esophagus.Cancers (Basel). 2022 Sep 30;14(19):4789. doi: 10.3390/cancers14194789. Cancers (Basel). 2022. PMID: 36230712 Free PMC article.
-
TROP2 (trophoblast cell-surface antigen 2): a drug target for breast cancer.Expert Opin Ther Targets. 2022 Jul;26(7):593-602. doi: 10.1080/14728222.2022.2113513. Epub 2022 Aug 19. Expert Opin Ther Targets. 2022. PMID: 35962580
Cited by
-
Advances in Trop-2 targeted antibody-drug conjugates for breast cancer: mechanisms, clinical applications, and future directions.Front Immunol. 2024 Nov 1;15:1495675. doi: 10.3389/fimmu.2024.1495675. eCollection 2024. Front Immunol. 2024. PMID: 39555080 Free PMC article. Review.
References
-
- Giaquinto AN, Sung H, Miller KD, et al. Breast Cancer Stat 2022 CA: cancer J Clin. 2022;72(6):524–41. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

