This is a preprint.
Secreted chemokines reveal diverse inflammatory and degenerative processes in the intervertebral disc of the STZ-HFD mouse model of Type 2 diabetes
- PMID: 39131361
- PMCID: PMC11312574
- DOI: 10.1101/2024.07.31.605332
Secreted chemokines reveal diverse inflammatory and degenerative processes in the intervertebral disc of the STZ-HFD mouse model of Type 2 diabetes
Abstract
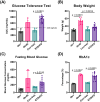
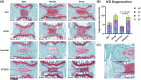
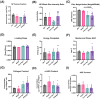
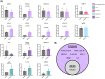
The chronic inflammation present in type 2 diabetes causes many chronic inflammatory comorbidities, including cardiovascular, renal, and neuropathic complications. Type 2 diabetes is also associated with a number of spinal pathologies, including intervertebral disc (IVD) degeneration and chronic neck and back pain. Although confounding factors such as obesity are thought to increase the loads to the musculoskeletal system and subsequent degeneration, studies have shown that even after adjusting age, body mass index, and genetics (e.g. twins), patients with diabetes suffer from disproportionately more IVD degeneration and back pain. Yet the tissue-specific responses of the IVD during diabetes remains relatively unknown. We hypothesize that chronic diabetes fosters a proinflammatory microenvironment within the IVD that accelerates degeneration and increases susceptibility to painful disorders. To test this hypothesis, we evaluated two commonly used mouse models of diabetes - the leptin-receptor deficient mouse (db/db) and the chronic high-fat diet in mice with impaired beta-cell function (STZ-HFD). The db/db is a genetic model that spontaneous develop diabetes through hyperphagia, while the STZ-HFD mouse first exhibits rapid obesity development under HFD and pronounced insulin resistance following streptozotocin administration. Both animal models were allowed to develop sustained diabetes for at least twelve weeks, as defined by elevated hemoglobin A1C, hyperglycemia, and glucose intolerance. Following the twelve-week period, the IVDs were extracted in quantified in several measures including tissue-specific secreted cytokines, viscoelastic mechanical behavior, structural composition, and histopathologic degeneration. Although there were no differences in mechanical function or the overall structure of the IVD, the STZ-HFD IVDs were more degenerated. More notably, the STZ-HFD model shows a significantly higher fold increase for eight cytokines: CXCL2, CCL2, CCL3, CCL4, CCL12 (monocyte/macrophage associated), IL-2, CXCL9 (T-cell associated), and CCL5 (pleiotropic). Correlative network analyses revealed that the expression of cytokines differentially regulated between the db/db and the STZ-HFD models. Moreover, the STZ-HFD contained a fragmented and modular cytokine network, indicating greater complexities in the regulatory network. Taken together, the STZ-HFD model of type 2 diabetes may better recapitulate the complexities of the chronic inflammatory processes in the IVD during diabetes.
Keywords: chronic inflammatory cytokines; intervertebral disc degeneration; leptin receptor deficiency; streptozotocin-high-fat-diet; type 2 diabetes.
Conflict of interest statement
Conflicts of Interest Authors declare no conflict of interest.
Figures


Similar articles
-
Leptin signaling and the intervertebral disc: Sex dependent effects of leptin receptor deficiency and Western diet on the spine in a type 2 diabetes mouse model.PLoS One. 2020 May 6;15(5):e0227527. doi: 10.1371/journal.pone.0227527. eCollection 2020. PLoS One. 2020. PMID: 32374776 Free PMC article.
-
Inflammatory profiles in canine intervertebral disc degeneration.BMC Vet Res. 2016 Jan 13;12:10. doi: 10.1186/s12917-016-0635-6. BMC Vet Res. 2016. PMID: 26757881 Free PMC article.
-
Anti-inflammatory effects of interleukin-4 on intervertebral disc cells.Spine J. 2020 Jan;20(1):60-68. doi: 10.1016/j.spinee.2019.06.025. Epub 2019 Jun 29. Spine J. 2020. PMID: 31265894
-
Role of cytokines in intervertebral disc degeneration: pain and disc content.Nat Rev Rheumatol. 2014 Jan;10(1):44-56. doi: 10.1038/nrrheum.2013.160. Epub 2013 Oct 29. Nat Rev Rheumatol. 2014. PMID: 24166242 Free PMC article. Review.
-
Bioenergetic dysfunction in the pathogenesis of intervertebral disc degeneration.Pharmacol Res. 2024 Apr;202:107119. doi: 10.1016/j.phrs.2024.107119. Epub 2024 Feb 28. Pharmacol Res. 2024. PMID: 38417775 Review.
References
-
- Alpantaki K., Kampouroglou A., Koutserimpas C., Effraimidis G. and Hadjipavlou A. (2019). Diabetes mellitus as a risk factor for intervertebral disc degeneration: a critical review. Eur. Spine J. 28, 2129–2144. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
