Protocol for the isolation and purification of endoplasmic reticulum-plasma membrane junctions from the mouse brain
- PMID: 39126654
- PMCID: PMC11369423
- DOI: 10.1016/j.xpro.2024.103253
Protocol for the isolation and purification of endoplasmic reticulum-plasma membrane junctions from the mouse brain
Abstract
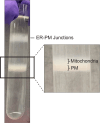
Dynamic communication between intracellular organelles often takes place at specialized membrane contact sites that form between their membranes. Here we detail a procedure for the purification of endoplasmic reticulum-plasma membrane (ER-PM) junctions from the mouse brain. We describe steps for homogenizing isolated brain hemispheres and sequential centrifugation to remove the nuclear fraction from the other membrane fractions. We then detail procedures for separating the resulting crude membrane fractions by sucrose density gradients and purifying into their respective pellets. For complete details on the use and execution of this protocol, please refer to Weesner et al.1.
Keywords: Cell Membrane; Cell separation/fractionation; Neuroscience.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
















Similar articles
-
Isolation of plasma membrane-associated membranes from rat liver.Nat Protoc. 2014 Feb;9(2):312-22. doi: 10.1038/nprot.2014.016. Epub 2014 Jan 16. Nat Protoc. 2014. PMID: 24434800
-
Isolation of Mitochondria-Associated Membranes (MAM) from Mouse Brain Tissue.Methods Mol Biol. 2017;1567:53-68. doi: 10.1007/978-1-4939-6824-4_5. Methods Mol Biol. 2017. PMID: 28276013
-
Purification of yeast membranes and organelles by sucrose density gradient centrifugation.Methods Mol Biol. 2008;457:141-9. doi: 10.1007/978-1-59745-261-8_10. Methods Mol Biol. 2008. PMID: 19066024
-
ER-PM connections: sites of information transfer and inter-organelle communication.Curr Opin Cell Biol. 2013 Aug;25(4):434-42. doi: 10.1016/j.ceb.2013.02.020. Epub 2013 Mar 20. Curr Opin Cell Biol. 2013. PMID: 23522446 Free PMC article. Review.
-
Endoplasmic Reticulum-Plasma Membrane Associations:Structures and Functions.Annu Rev Cell Dev Biol. 2016 Oct 6;32:279-301. doi: 10.1146/annurev-cellbio-111315-125024. Epub 2016 Jun 1. Annu Rev Cell Dev Biol. 2016. PMID: 27298092 Review.
References
-
- Weesner J.A., Annunziata I., van de Vlekkert D., Robinson C.G., Campos Y., Mishra A., Fremuth L.E., Gomero E., Hu H., d'Azzo A. Altered GM1 catabolism affects NMDAR-mediated Ca(2+) signaling at ER-PM junctions and increases synaptic spine formation in a GM1-gangliosidosis model. Cell Rep. 2024;43 doi: 10.1016/j.celrep.2024.114117. - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

