Accidental Encounter of Repair Intermediates in Alkylated DNA May Lead to Double-Strand Breaks in Resting Cells
- PMID: 39125763
- PMCID: PMC11311527
- DOI: 10.3390/ijms25158192
Accidental Encounter of Repair Intermediates in Alkylated DNA May Lead to Double-Strand Breaks in Resting Cells
Abstract
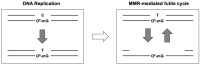
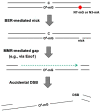
In clinics, chemotherapy is often combined with surgery and radiation to increase the chances of curing cancers. In the case of glioblastoma (GBM), patients are treated with a combination of radiotherapy and TMZ over several weeks. Despite its common use, the mechanism of action of the alkylating agent TMZ has not been well understood when it comes to its cytotoxic effects in tumor cells that are mostly non-dividing. The cellular response to alkylating DNA damage is operated by an intricate protein network involving multiple DNA repair pathways and numerous checkpoint proteins that are dependent on the type of DNA lesion, the cell type, and the cellular proliferation state. Among the various alkylating damages, researchers have placed a special on O6-methylguanine (O6-mG). Indeed, this lesion is efficiently removed via direct reversal by O6-methylguanine-DNA methyltransferase (MGMT). As the level of MGMT expression was found to be directly correlated with TMZ efficiency, O6-mG was identified as the critical lesion for TMZ mode of action. Initially, the mode of action of TMZ was proposed as follows: when left on the genome, O6-mG lesions form O6-mG: T mispairs during replication as T is preferentially mis-inserted across O6-mG. These O6-mG: T mispairs are recognized and tentatively repaired by a post-replicative mismatched DNA correction system (i.e., the MMR system). There are two models (futile cycle and direct signaling models) to account for the cytotoxic effects of the O6-mG lesions, both depending upon the functional MMR system in replicating cells. Alternatively, to explain the cytotoxic effects of alkylating agents in non-replicating cells, we have proposed a "repair accident model" whose molecular mechanism is dependent upon crosstalk between the MMR and the base excision repair (BER) systems. The accidental encounter between these two repair systems will cause the formation of cytotoxic DNA double-strand breaks (DSBs). In this review, we summarize these non-exclusive models to explain the cytotoxic effects of alkylating agents and discuss potential strategies to improve the clinical use of alkylating agents.
Keywords: DNA damages; DNA double-strand breaks; DNA repairs; chemotherapy; repair accident model.
Conflict of interest statement
R.P.F. and S.F. are co-founders and consultants at the company, bioHalosis. The company had no role in the design of this study; in the collection, analysis, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results. The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflicts of interest.
Figures
Similar articles
-
Double-strand breaks: When DNA repair events accidentally meet.DNA Repair (Amst). 2022 Apr;112:103303. doi: 10.1016/j.dnarep.2022.103303. Epub 2022 Feb 19. DNA Repair (Amst). 2022. PMID: 35219626 Free PMC article. Review.
-
Crosstalk between repair pathways elicits double-strand breaks in alkylated DNA and implications for the action of temozolomide.Elife. 2021 Jul 8;10:e69544. doi: 10.7554/eLife.69544. Elife. 2021. PMID: 34236314 Free PMC article.
-
Overcoming temozolomide resistance in glioblastoma via dual inhibition of NAD+ biosynthesis and base excision repair.Cancer Res. 2011 Mar 15;71(6):2308-17. doi: 10.1158/0008-5472.CAN-10-3213. Cancer Res. 2011. PMID: 21406402 Free PMC article.
-
Temozolomide: mechanisms of action, repair and resistance.Curr Mol Pharmacol. 2012 Jan;5(1):102-14. doi: 10.2174/1874467211205010102. Curr Mol Pharmacol. 2012. PMID: 22122467
-
MGMT: key node in the battle against genotoxicity, carcinogenicity and apoptosis induced by alkylating agents.DNA Repair (Amst). 2007 Aug 1;6(8):1079-99. doi: 10.1016/j.dnarep.2007.03.008. Epub 2007 May 7. DNA Repair (Amst). 2007. PMID: 17485253 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

