Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments
- PMID: 39125732
- PMCID: PMC11311339
- DOI: 10.3390/ijms25158163
Balancing the Scales: The Dual Role of Interleukins in Bone Metastatic Microenvironments
Abstract
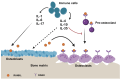
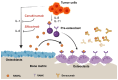
Bone metastases, a common and debilitating consequence of advanced cancers, involve a complex interplay between malignant cells and the bone microenvironment. Central to this interaction are interleukins (ILs), a group of cytokines with critical roles in immune modulation and inflammation. This review explores the dualistic nature of pro-inflammatory and anti-inflammatory interleukins in bone metastases, emphasizing their molecular mechanisms, pathological impacts, and therapeutic potential. Pro-inflammatory interleukins, such as IL-1, IL-6, and IL-8, have been identified as key drivers in promoting osteoclastogenesis, tumor proliferation, and angiogenesis. These cytokines create a favorable environment for cancer cell survival and bone degradation, contributing to the progression of metastatic lesions. Conversely, anti-inflammatory interleukins, including IL-4, IL-10, and IL-13, exhibit protective roles by modulating immune responses and inhibiting osteoclast activity. Understanding these opposing effects is crucial for developing targeted therapies aimed at disrupting the pathological processes in bone metastases. Key signaling pathways, including NF-κB, JAK/STAT, and MAPK, mediate the actions of these interleukins, influencing tumor cell survival, immune cell recruitment, and bone remodeling. Targeting these pathways presents promising therapeutic avenues. Current treatment strategies, such as the use of denosumab, tocilizumab, and emerging agents like bimekizumab and ANV419, highlight the potential of interleukin-targeted therapies in mitigating bone metastases. However, challenges such as therapeutic resistance, side effects, and long-term efficacy remain significant hurdles. This review also addresses the potential of interleukins as diagnostic and prognostic biomarkers, offering insights into patient stratification and personalized treatment approaches. Interleukins have multifaceted roles that depend on the context, including the environment, cell types, and cellular interactions. Despite substantial progress, gaps in research persist, particularly regarding the precise mechanisms by which interleukins influence the bone metastatic niche and their broader clinical implications. While not exhaustive, this overview underscores the critical roles of interleukins in bone metastases and highlights the need for continued research to fully elucidate their complex interactions and therapeutic potential. Addressing these gaps will be essential for advancing our understanding and treatment of bone metastases in cancer patients.
Keywords: anti-inflammatory; bone metastasis; interleukins; pro-inflammatory; therapy; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Crosstalk of Inflammatory Cytokines within the Breast Tumor Microenvironment.Int J Mol Sci. 2023 Feb 16;24(4):4002. doi: 10.3390/ijms24044002. Int J Mol Sci. 2023. PMID: 36835413 Free PMC article. Review.
-
From Tumor to Bone: Growth Factor Receptors as Key Players in Cancer Metastasis.Front Biosci (Landmark Ed). 2024 May 13;29(5):184. doi: 10.31083/j.fbl2905184. Front Biosci (Landmark Ed). 2024. PMID: 38812320 Review.
-
Interleukins as Mediators of the Tumor Cell-Bone Cell Crosstalk during the Initiation of Breast Cancer Bone Metastasis.Int J Mol Sci. 2021 Mar 12;22(6):2898. doi: 10.3390/ijms22062898. Int J Mol Sci. 2021. PMID: 33809315 Free PMC article. Review.
-
Intersecting Paths: Unraveling the Complex Journey of Cancer to Bone Metastasis.Biomedicines. 2024 May 13;12(5):1075. doi: 10.3390/biomedicines12051075. Biomedicines. 2024. PMID: 38791037 Free PMC article. Review.
-
Dendritic cells-derived interferon-λ1 ameliorated inflammatory bone destruction through inhibiting osteoclastogenesis.Cell Death Dis. 2020 Jun 2;11(6):414. doi: 10.1038/s41419-020-2612-z. Cell Death Dis. 2020. PMID: 32488049 Free PMC article.
References
-
- Sethakorn N., Heninger E., Sánchez-de-Diego C., Ding A.B., Yada R.C., Kerr S.C., Kosoff D., Beebe D.J., Lang J.M. Advancing Treatment of Bone Metastases through Novel Translational Approaches Targeting the Bone Microenvironment. Cancers. 2022;14:757. doi: 10.3390/cancers14030757. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

