Nature of the Association between Rheumatoid Arthritis and Cervical Cancer and Its Potential Therapeutic Implications
- PMID: 39125448
- PMCID: PMC11314534
- DOI: 10.3390/nu16152569
Nature of the Association between Rheumatoid Arthritis and Cervical Cancer and Its Potential Therapeutic Implications
Abstract
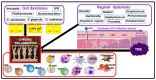
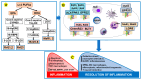
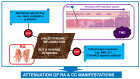
It is now established that patients with rheumatoid arthritis (RA) have an increased risk of developing cervical cancer (CC) or its precursor, cervical intraepithelial neoplasia (CIN). However, the underlying mechanisms of this association have not been elucidated. RA is characterized by unresolved chronic inflammation. It is suggested that human papillomavirus (HPV) infection in RA patients exacerbates inflammation, increasing the risk of CC. The tumor microenvironment in RA patients with CC is also marked by chronic inflammation, which aggravates the manifestations of both conditions. Gut and vaginal dysbiosis are also considered potential mechanisms that contribute to the chronic inflammation and aggravation of RA and CC manifestations. Numerous clinical and pre-clinical studies have demonstrated the beneficial effects of various nutritional approaches to attenuate chronic inflammation, including polyunsaturated fatty acids and their derivatives, specialized pro-resolving mediators (SPMs), probiotics, prebiotics, and certain diets. We believe that successful resolution of chronic inflammation and correction of dysbiosis, in combination with current anti-RA and anti-CC therapies, is a promising therapeutic approach for RA and CC. This approach could also reduce the risk of CC development in HPV-infected RA patients.
Keywords: HPV-infection; chronic inflammation; dysbiosis; nutrition; specialized pro-resolving mediators.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Therapeutic potential of probiotics in gut microbial homeostasis and Rheumatoid arthritis.Int Immunopharmacol. 2024 Aug 20;137:112501. doi: 10.1016/j.intimp.2024.112501. Epub 2024 Jun 16. Int Immunopharmacol. 2024. PMID: 38885604 Review.
-
Guidelines for Cervical Cancer Screening in Immunosuppressed Women Without HIV Infection.J Low Genit Tract Dis. 2019 Apr;23(2):87-101. doi: 10.1097/LGT.0000000000000468. J Low Genit Tract Dis. 2019. PMID: 30907775
-
Rheumatoid arthritis and the intestinal microbiome: probiotics as a potential therapy.Front Immunol. 2024 Mar 6;15:1331486. doi: 10.3389/fimmu.2024.1331486. eCollection 2024. Front Immunol. 2024. PMID: 38510244 Free PMC article. Review.
-
Modification of Gut Microbiota in Inflammatory Arthritis: Highlights and Future Challenges.Curr Rheumatol Rep. 2021 Jul 3;23(8):67. doi: 10.1007/s11926-021-01031-9. Curr Rheumatol Rep. 2021. PMID: 34218340 Review.
-
The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next?Microbiome. 2016 Nov 1;4(1):58. doi: 10.1186/s40168-016-0203-0. Microbiome. 2016. PMID: 27802830 Free PMC article. Review.
References
-
- Kim S.C., Glynn R.J., Giovannucci E., Hernández-Díaz S., Liu J., Feldman S., Karlson E.W., Schneeweiss S., Solomon D.H. Risk of High-Grade Cervical Dysplasia and Cervical Cancer in Women with Systemic Inflammatory Diseases: A Population-Based Cohort Study. Ann. Rheum. Dis. 2015;74:1360–1367. doi: 10.1136/annrheumdis-2013-204993. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

