Multi-omics analysis of antiviral interactions of Elizabethkingia anophelis and Zika virus
- PMID: 39122799
- PMCID: PMC11315927
- DOI: 10.1038/s41598-024-68898-3
Multi-omics analysis of antiviral interactions of Elizabethkingia anophelis and Zika virus
Abstract
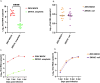
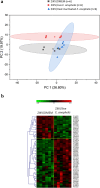
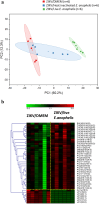
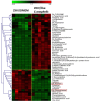
The microbial communities residing in the mosquito midgut play a key role in determining the outcome of mosquito pathogen infection. Elizabethkingia anophelis, originally isolated from the midgut of Anopheles gambiae possess a broad-spectrum antiviral phenotype, yet a gap in knowledge regarding the mechanistic basis of its interaction with viruses exists. The current study aims to identify pathways and genetic factors linked to E. anophelis antiviral activity. The understanding of E. anophelis antiviral mechanism could lead to novel transmission barrier tools to prevent arboviral outbreaks. We utilized a non-targeted multi-omics approach, analyzing extracellular lipids, proteins, metabolites of culture supernatants coinfected with ZIKV and E. anophelis. We observed a significant decrease in arginine and phenylalanine levels, metabolites that are essential for viral replication and progression of viral infection. This study provides insights into the molecular basis of E. anophelis antiviral phenotype. The findings lay a foundation for in-depth mechanistic studies.
Keywords: Elizabethkingia anopheles; Antiviral interactions; Multi-omics analysis; Zika virus.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Zika virus and temperature modulate Elizabethkingia anophelis in Aedes albopictus.Parasit Vectors. 2021 Nov 12;14(1):573. doi: 10.1186/s13071-021-05069-7. Parasit Vectors. 2021. PMID: 34772442 Free PMC article.
-
Elizabethkingia anophelis: molecular manipulation and interactions with mosquito hosts.Appl Environ Microbiol. 2015 Mar;81(6):2233-43. doi: 10.1128/AEM.03733-14. Epub 2015 Jan 16. Appl Environ Microbiol. 2015. PMID: 25595771 Free PMC article.
-
Comparative genomic analysis of malaria mosquito vector-associated novel pathogen Elizabethkingia anophelis.Genome Biol Evol. 2014 May 6;6(5):1158-65. doi: 10.1093/gbe/evu094. Genome Biol Evol. 2014. PMID: 24803570 Free PMC article.
-
Zika virus: An updated review of competent or naturally infected mosquitoes.PLoS Negl Trop Dis. 2017 Nov 16;11(11):e0005933. doi: 10.1371/journal.pntd.0005933. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29145400 Free PMC article. Review.
-
ZIKA virus entry mechanisms in human cells.Infect Genet Evol. 2019 Apr;69:22-29. doi: 10.1016/j.meegid.2019.01.018. Epub 2019 Jan 15. Infect Genet Evol. 2019. PMID: 30658214 Review.
References
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

