Fast on-rates of chimeric antigen receptors enhance the sensitivity to peptide MHC via antigen rebinding
- PMID: 39122001
- PMCID: PMC11407991
- DOI: 10.1016/j.jbc.2024.107651
Fast on-rates of chimeric antigen receptors enhance the sensitivity to peptide MHC via antigen rebinding
Erratum in
-
Correction: Fast on-rates of chimeric antigen receptors enhance the sensitivity to peptide MHC via antigen rebinding.J Biol Chem. 2024 Dec;300(12):108008. doi: 10.1016/j.jbc.2024.108008. Epub 2024 Dec 9. J Biol Chem. 2024. PMID: 39657330 Free PMC article. No abstract available.
Abstract
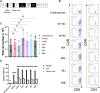
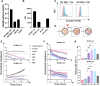
Chimeric antigen receptor (CAR) is a synthetic receptor that induces T cell-mediated lysis of abnormal cells. As cancer driver proteins are present at low levels on the cell surface, they can cause weak CAR reactivity, resulting in antigen sensitivity defects and consequently limited therapeutic efficacy. Although affinity maturation enhances the efficacy of CAR-T cell therapy, it causes off-target cross-reactions resulting in adverse effects. Preferentially expressed antigen in melanoma (PRAME) is an intracellular oncoprotein that is overexpressed in various tumors and restricted in normal tissues, except the testis. Therefore, PRAME could be an ideal target for cancer immunotherapy. In this study, we developed an experimental CAR system comprising six single-chain variable fragments that specifically recognizes the PRAMEp301/HLA-A∗24:02 complex. Cell-mediated cytotoxicity was demonstrated using a panel of CARs with a wide range of affinities (KD = 10-10-10-7 M) and affinity modulation. CAR-T cells with fast on-rates enhance antigen sensitivity by accelerating the killing rates of these cells. Alanine scanning data demonstrated the potential of genetically engineered CARs to reduce the risk of cross-reactivity, even among CARs with high affinities. Given the correlation between on-rates and dwell time that occurs in rebinding and cell-mediated cytotoxicity, it is proposed that CAR-binding characteristics, including on-rate, play a pivotal role in the lytic capacity of peptide-major histocompatibility complex-targeting CAR-T cells, thus facilitating the development of strategies whereby genetically engineered CARs target intracellular antigens in cancer cells to lyse the cells.
Keywords: T cell; binding affinity; cancer; cell engineering; chimeric antigen receptor; immunotherapy; major histocompatibility complex (MHC); peptides; receptor.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest Hiroyuki Hiratsuka and Yasushi Akahori have filed provisional patent applications for the sequence data of WT98 (WO 2022124282-A/1–WO 2022124282-A/8) and WT163 (JP2024–115711). The late Hiroshi Shiku was involved in the patent application for WT98. Part of this research was funded by a cooperative research grant from Sysmex Corporation. Shingo Maeta and Yuriko Egashira are affiliated with Sysmex Corporation.
Figures


Similar articles
-
Defining the optimum strategy for identifying adults and children with coeliac disease: systematic review and economic modelling.Health Technol Assess. 2022 Oct;26(44):1-310. doi: 10.3310/ZUCE8371. Health Technol Assess. 2022. PMID: 36321689 Free PMC article.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article.
-
Antioxidants for female subfertility.Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Aug 27;8:CD007807. doi: 10.1002/14651858.CD007807.pub4 PMID: 28752910 Free PMC article. Updated. Review.
References
-
- Labanieh L., Mackall C.L. CAR immune cells: design principles, resistance and the next generation. Nature. 2023;614:635–648. - PubMed
-
- Purbhoo M.A., Sutton D.H., Brewer J.E., Mullings R.E., Hill M.E., Mahon T.M., et al. Quantifying and imaging NY-ESO-1/LAGE-1-derived epitopes on tumor cells using high affinity T cell receptors. J. Immunol. 2006;176:7308–7316. - PubMed
-
- Gudipati V., Rydzek J., Doel-Perez I., Goncalves V.D.R., Scharf L., Konigsberger S., et al. Inefficient CAR-proximal signaling blunts antigen sensitivity. Nat. Immunol. 2020;21:848–856. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

