Durvalumab plus platinum-etoposide chemotherapy for extensive-stage small cell lung cancer: a retrospective real-world study
- PMID: 39118881
- PMCID: PMC11304155
- DOI: 10.21037/tlcr-24-128
Durvalumab plus platinum-etoposide chemotherapy for extensive-stage small cell lung cancer: a retrospective real-world study
Abstract
Background: Immune checkpoint inhibitor plus platinum-etoposide (PE) improved overall survival (OS) in patients with extensive-stage small cell lung cancer (ES-SCLC). While the CASPIAN trial demonstrated the efficacy of durvalumab plus PE, the clinical trial results may not be representative of the general, real-world population because clinical trials often have strict inclusion and exclusion criteria. We herein report the efficacy and safety of durvalumab plus PE in patients with ES-SCLC in real-world, clinical practice.
Methods: The present, monocentric, retrospective study evaluated patients with ES-SCLC or recurrent, limited-stage SCLC who received durvalumab plus PE between September 2020 and February 2023. The efficacy and incidence of adverse events (AEs) were also evaluated.
Results: The study included 40 patients, of whom 17 were elderly (age >70 years), and 15 had performance status (PS) 2 or 3. The median follow-up time was 13.0 months [95% confidence interval (CI): 8.0-22.2 months]. The objective response rate was 80.0% (95% CI: 63.1-91.6%), and the disease control rate was 88.6% (95% CI: 73.3-96.8%). The median progression-free survival (PFS) was 5.9 months (95% CI: 4.9-6.9), and the median OS was 25.4 months (95% CI: 4.6-46.2). Factors such as advanced age, poor PS, and presence of brain metastases were not associated with lower PFS and OS. Twenty-six patients (65.0%) experienced grade 3 or higher AEs, mainly hematological toxicity. AEs leading to treatment discontinuation occurred in three patients (8%).
Conclusions: Durvalumab plus PE in patients with ES-SCLC showed good efficacy and safety according to our real-world data, suggesting that this treatment is well tolerated in clinical practice, even in elderly patients and those with poor PS.
Keywords: CASPIAN; Small cell lung cancer (SCLC); durvalumab; immune checkpoint inhibitor (ICI); platinum-etoposide (PE).
2024 Translational Lung Cancer Research. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://tlcr.amegroups.com/article/view/10.21037/tlcr-24-128/coif). K.W. received payment or honoraria for lectures and presentations from Amgen, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Chugai Pharmaceutical, Merck Biopharma, MSD, Novartis, Ono Pharmaceutical, Riken Genesis, Sysmex Corporation and Takeda. M.S. received grants or contracts from Taiho Pharmaceutical, Chugai Pharmaceutical, Eli Lilly, Nippon Kayaku, Kyowa Hakko Kirin and payment or honoraria for lectures and presentations from AstraZeneca, MSD, Chugai Pharmaceutical, Taiho Pharmaceutical, Eli Lilly, Ono Pharmaceutical, Bristol-Myers Squibb, Nippon Boehringer Ingelheim, Pfizer, Novartis, Takeda, Kyowa Hakko Kirin, Nippon Kayaku, Daiichi-Sankyo Company, Merck Biopharma, Amgen. Y.H. received payment or honoraria for lectures and presentations from AstraZeneca, Eli Lilly Japan, Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol-Myers Squibb, Kyowa Hakko Kirin, Nihon Kayaku, Takeda, Eisai, Novartis and Pfizer. The other author has no conflicts of interest to declare.
Figures
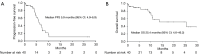

Similar articles
-
Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial.Lancet. 2019 Nov 23;394(10212):1929-1939. doi: 10.1016/S0140-6736(19)32222-6. Epub 2019 Oct 4. Lancet. 2019. PMID: 31590988 Clinical Trial.
-
A phase II study of carboplatin and etoposide plus durvalumab for previously untreated extensive-stage small-cell lung cancer (ES-SCLC) patients with a poor performance status (PS): NEJ045A study protocol.BMC Cancer. 2022 Nov 4;22(1):1135. doi: 10.1186/s12885-022-10222-1. BMC Cancer. 2022. PMID: 36333680 Free PMC article.
-
Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial.Lancet Oncol. 2021 Jan;22(1):51-65. doi: 10.1016/S1470-2045(20)30539-8. Epub 2020 Dec 4. Lancet Oncol. 2021. PMID: 33285097 Clinical Trial.
-
FDA Approval Summary: Atezolizumab and Durvalumab in Combination with Platinum-Based Chemotherapy in Extensive Stage Small Cell Lung Cancer.Oncologist. 2021 May;26(5):433-438. doi: 10.1002/onco.13752. Epub 2021 Mar 25. Oncologist. 2021. PMID: 33687763 Free PMC article. Review.
-
Durvalumab: A Review in Extensive-Stage SCLC.Target Oncol. 2021 Nov;16(6):857-864. doi: 10.1007/s11523-021-00843-0. Epub 2021 Nov 3. Target Oncol. 2021. PMID: 34731446 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
