On the generation of force required for actin-based motility
- PMID: 39117762
- PMCID: PMC11310465
- DOI: 10.1038/s41598-024-69422-3
On the generation of force required for actin-based motility
Abstract
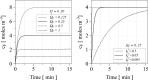
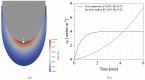
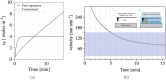
The fundamental question of how forces are generated in a motile cell, a lamellipodium, and a comet tail is the subject of this note. It is now well established that cellular motility results from the polymerization of actin, the most abundant protein in eukaryotic cells, into an interconnected set of filaments. We portray this process in a continuum mechanics framework, claiming that polymerization promotes a mechanical swelling in a narrow zone around the nucleation loci, which ultimately results in cellular or bacterial motility. To this aim, a new paradigm in continuum multi-physics has been designed, departing from the well-known theory of Larché-Cahn chemo-transport-mechanics. In this note, we set up the theory of network growth and compare the outcomes of numerical simulations with experimental evidence.
Keywords: Actin-based motility; Chemo-transport-mechanics; Continuum mechanics; Finite elements; High performance computing.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


Similar articles
-
Amplification of actin polymerization forces.J Cell Biol. 2016 Mar 28;212(7):763-6. doi: 10.1083/jcb.201512019. Epub 2016 Mar 21. J Cell Biol. 2016. PMID: 27002174 Free PMC article.
-
Network heterogeneity regulates steering in actin-based motility.Nat Commun. 2017 Sep 21;8(1):655. doi: 10.1038/s41467-017-00455-1. Nat Commun. 2017. PMID: 28935896 Free PMC article.
-
Simulation of cell motility that reproduces the force-velocity relationship.Proc Natl Acad Sci U S A. 2010 May 18;107(20):9141-6. doi: 10.1073/pnas.1002538107. Epub 2010 May 3. Proc Natl Acad Sci U S A. 2010. PMID: 20439759 Free PMC article.
-
Actin polymerization machinery: the finish line of signaling networks, the starting point of cellular movement.Cell Mol Life Sci. 2005 May;62(9):955-70. doi: 10.1007/s00018-004-4472-6. Cell Mol Life Sci. 2005. PMID: 15868099 Review.
-
The comings and goings of actin: coupling protrusion and retraction in cell motility.Curr Opin Cell Biol. 2005 Oct;17(5):517-23. doi: 10.1016/j.ceb.2005.08.004. Curr Opin Cell Biol. 2005. PMID: 16099152 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

