A chemoenzymatic method for simultaneous profiling N- and O-glycans on glycoproteins using one-pot format
- PMID: 39116882
- PMCID: PMC11384086
- DOI: 10.1016/j.crmeth.2024.100834
A chemoenzymatic method for simultaneous profiling N- and O-glycans on glycoproteins using one-pot format
Abstract
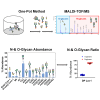
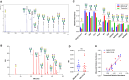
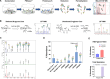
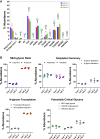
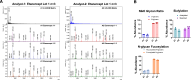
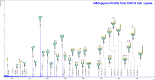
Glycosylation is generally characterized and controlled as a critical quality attribute for therapeutic glycoproteins because glycans can impact protein drug-product efficacy, half-life, stability, and safety. Analytical procedures to characterize N-glycans are relatively well established, but the characterization of O-glycans is challenging due to the complex workflows and lack of enzymatic tools. Here, we present a simplified chemoenzymatic method to simultaneously profile N- and O-glycans from the same sample using a one-pot format by mass spectrometry (MS). N-glycans were first released by PNGase F, followed by O-glycopeptide generation by proteinase K, selective N-glycan reduction, and O-glycan release by β-elimination during permethylation of both N- and O-glycans. Glycan structural assignments and determination of N- to O-glycan ratio was obtained from the one-pot mass spectra. The streamlined, one-pot method is a reliable approach that will facilitate advanced characterizations for quality assessments of therapeutic glycoproteins.
Keywords: CP: biotechnology; N-glycans; O-glycans; biotechnology; glycan analysis; glycomics; mass spectrometry; protein drugs.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures

Similar articles
-
PNGase F-mediated incorporation of (18)O into glycans for relative glycan quantitation.Analyst. 2015 Feb 21;140(4):1082-9. doi: 10.1039/c4an02073a. Analyst. 2015. PMID: 25521995
-
Analysis of protein glycosylation by mass spectrometry.Nat Protoc. 2007;2(7):1585-602. doi: 10.1038/nprot.2007.227. Nat Protoc. 2007. PMID: 17585300
-
Quantitative O-glycomics based on improvement of the one-pot method for nonreductive O-glycan release and simultaneous stable isotope labeling with 1-(d0/d5)phenyl-3-methyl-5-pyrazolone followed by mass spectrometric analysis.J Proteomics. 2017 Jan 6;150:18-30. doi: 10.1016/j.jprot.2016.08.012. Epub 2016 Aug 29. J Proteomics. 2017. PMID: 27585995
-
Decoding of O-Linked Glycosylation by Mass Spectrometry.Biochemistry. 2017 Mar 7;56(9):1218-1226. doi: 10.1021/acs.biochem.6b01244. Epub 2017 Feb 27. Biochemistry. 2017. PMID: 28196325 Review.
-
Analysis of N- and O-linked glycans from glycoproteins using MALDI-TOF mass spectrometry.Methods Mol Biol. 2009;534:5-21. doi: 10.1007/978-1-59745-022-5_1. Methods Mol Biol. 2009. PMID: 19277556 Review.
References
-
- Mimura Y., Katoh T., Saldova R., O'Flaherty R., Izumi T., Mimura-Kimura Y., Utsunomiya T., Mizukami Y., Yamamoto K., Matsumoto T., Rudd P.M. Glycosylation engineering of therapeutic IgG antibodies: challenges for the safety, functionality and efficacy. Protein Cell. 2018;9:47–62. doi: 10.1007/s13238-017-0433-3. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

