Evaluation of a novel intramuscular prime/intranasal boost vaccination strategy against influenza in the pig model
- PMID: 39116029
- PMCID: PMC11309389
- DOI: 10.1371/journal.ppat.1012393
Evaluation of a novel intramuscular prime/intranasal boost vaccination strategy against influenza in the pig model
Abstract
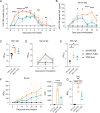
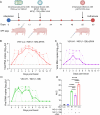
Live-attenuated influenza vaccines (LAIV) offer advantages over the commonly used inactivated split influenza vaccines. However, finding the optimal balance between sufficient attenuation and immunogenicity has remained a challenge. We recently developed an alternative LAIV based on the 2009 pandemic H1N1 virus with a truncated NS1 protein and lacking PA-X protein expression (NS1(1-126)-ΔPAX). This virus showed a blunted replication and elicited a strong innate immune response. In the present study, we evaluated the efficacy of this vaccine candidate in the porcine animal model as a pertinent in vivo system. Immunization of pigs via the nasal route with the novel NS1(1-126)-ΔPAX LAIV did not cause disease and elicited a strong mucosal immune response that completely blocked replication of the homologous challenge virus in the respiratory tract. However, we observed prolonged shedding of our vaccine candidate from the upper respiratory tract. To improve LAIV safety, we developed a novel prime/boost vaccination strategy combining primary intramuscular immunization with a haemagglutinin-encoding propagation-defective vesicular stomatitis virus (VSV) replicon, followed by a secondary immunization with the NS1(1-126)-ΔPAX LAIV via the nasal route. This two-step immunization procedure significantly reduced LAIV shedding, increased the production of specific serum IgG, neutralizing antibodies, and Th1 memory cells, and resulted in sterilizing immunity against homologous virus challenge. In conclusion, our novel intramuscular prime/intranasal boost regimen interferes with virus shedding and transmission, a feature that will help combat influenza epidemics and pandemics.
Copyright: © 2024 Avanthay et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
RA, GZ, and AS filed a patent related to the intramuscular prime/intranasal boost vaccine described in this work. All other authors declare no competing interests.
Figures




Similar articles
-
Protection of pigs against pandemic swine origin H1N1 influenza A virus infection by hemagglutinin- or neuraminidase-expressing attenuated pseudorabies virus recombinants.Virus Res. 2015 Mar 2;199:20-30. doi: 10.1016/j.virusres.2015.01.009. Epub 2015 Jan 17. Virus Res. 2015. PMID: 25599604
-
Generation of DelNS1 Influenza Viruses: a Strategy for Optimizing Live Attenuated Influenza Vaccines.mBio. 2019 Sep 17;10(5):e02180-19. doi: 10.1128/mBio.02180-19. mBio. 2019. PMID: 31530680 Free PMC article.
-
Pre-existing influenza-specific nasal IgA or nasal viral infection does not affect live attenuated influenza vaccine immunogenicity in children.Clin Exp Immunol. 2021 Apr;204(1):125-133. doi: 10.1111/cei.13564. Epub 2021 Jan 13. Clin Exp Immunol. 2021. PMID: 33314126 Free PMC article. Clinical Trial.
-
Novel Approaches for The Development of Live Attenuated Influenza Vaccines.Viruses. 2019 Feb 22;11(2):190. doi: 10.3390/v11020190. Viruses. 2019. PMID: 30813325 Free PMC article. Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

