Taz/Tead1 Promotes Alternative Macrophage Activation and Kidney Fibrosis via Transcriptional Upregulation of Smad3
- PMID: 39108258
- PMCID: PMC11303051
- DOI: 10.1155/2024/9512251
Taz/Tead1 Promotes Alternative Macrophage Activation and Kidney Fibrosis via Transcriptional Upregulation of Smad3
Abstract
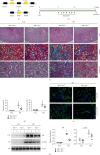
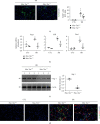
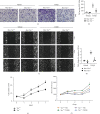
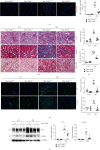
Macrophage alternative activation is involved in kidney fibrosis. Previous researches have documented that the transcriptional regulators Yes-associated protein (Yap)/transcriptional coactivator with PDZ-binding motif (Taz) are linked to organ fibrosis. However, limited knowledge exists regarding the function and mechanisms of their downstream molecules in regulating macrophage activation and kidney fibrosis. In this paper, we observed that the Hippo pathway was suppressed in macrophages derived from fibrotic kidneys in mice. Knockout of Taz or Tead1 in macrophages inhibited the alternative activation of macrophages and reduced kidney fibrosis. Additionally, by using bone marrow-derived macrophages (BMDMs), we investigated that knockout of Taz or Tead1 in macrophages impeded both cell proliferation and migration. Moreover, deletion of Tead1 reduces p-Smad3 and Smad3 abundance in macrophages. And chromatin immunoprecipitation (ChIP) assays showed that Tead1 could directly bind to the promoter region of Smad3. Collectively, these results indicate that Tead1 knockout in macrophages could reduce TGFβ1-induced phosphorylation Smad3 via transcriptional downregulation of Smad3, thus suppressing macrophage alternative activation and IRI-induced kidney fibrosis.
Copyright © 2024 Yizhi Ren et al.
Conflict of interest statement
This research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Figures



Similar articles
-
The signaling protein Wnt5a promotes TGFβ1-mediated macrophage polarization and kidney fibrosis by inducing the transcriptional regulators Yap/Taz.J Biol Chem. 2018 Dec 14;293(50):19290-19302. doi: 10.1074/jbc.RA118.005457. Epub 2018 Oct 17. J Biol Chem. 2018. PMID: 30333225 Free PMC article.
-
Deregulation of Hippo-TAZ pathway during renal injury confers a fibrotic maladaptive phenotype.FASEB J. 2018 May;32(5):2644-2657. doi: 10.1096/fj.201700722R. Epub 2018 Jan 3. FASEB J. 2018. PMID: 29298862 Free PMC article.
-
The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway.Kidney Int. 2013 Dec;84(6):1129-44. doi: 10.1038/ki.2013.272. Epub 2013 Jul 17. Kidney Int. 2013. PMID: 23868013
-
Neural transcription factor Pou4f1 promotes renal fibrosis via macrophage-myofibroblast transition.Proc Natl Acad Sci U S A. 2020 Aug 25;117(34):20741-20752. doi: 10.1073/pnas.1917663117. Epub 2020 Aug 11. Proc Natl Acad Sci U S A. 2020. PMID: 32788346 Free PMC article.
-
TGF-β/Smad3 signalling regulates the transition of bone marrow-derived macrophages into myofibroblasts during tissue fibrosis.Oncotarget. 2016 Feb 23;7(8):8809-22. doi: 10.18632/oncotarget.6604. Oncotarget. 2016. PMID: 26684242 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

