Translational Diffusion and Self-Association of an Intrinsically Disordered Protein κ-Casein Using NMR with Ultra-High Pulsed-Field Gradient and Time-Resolved FRET
- PMID: 39106061
- PMCID: PMC11331516
- DOI: 10.1021/acs.jpcb.4c03625
Translational Diffusion and Self-Association of an Intrinsically Disordered Protein κ-Casein Using NMR with Ultra-High Pulsed-Field Gradient and Time-Resolved FRET
Abstract
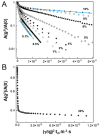
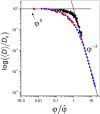
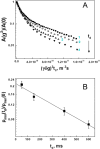
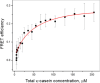
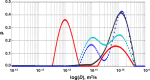
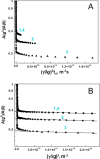
Much attention has been given to studying the translational diffusion of globular proteins, whereas the translational diffusion of intrinsically disordered proteins (IDPs) is less studied. In this study, we investigate the translational diffusion and how it is affected by the self-association of an IDP, κ-casein, using pulsed-field gradient nuclear magnetic resonance and time-resolved Förster resonance energy transfer. Using the analysis of the shape of diffusion attenuation and the concentration dependence of κ-casein diffusion coefficients and intermolecular interactions, we demonstrate that κ-casein exhibits continuous self-association. When the volume fraction of κ-casein is below 0.08, we observe that κ-casein self-association results in a macroscopic phase separation upon storage at 4 °C. At κ-casein volume fractions above 0.08, self-association leads to the formation of labile gel-like networks without subsequent macroscopic phase separation. Unlike α-casein, which shows a strong concentration dependence and extensive gel-like network formation, only one-third of κ-casein molecules participate in the gel network at a time, resulting in a more dynamic and less extensive structure. These findings highlight the unique association properties of κ-casein, contributing to a better understanding of its behavior under various conditions and its potential role in casein micelle formation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Effect of Intrinsic Disorder and Self-Association on the Translational Diffusion of Proteins: The Case of α-Casein.J Phys Chem B. 2017 Apr 13;121(14):2980-2988. doi: 10.1021/acs.jpcb.7b00772. Epub 2017 Mar 31. J Phys Chem B. 2017. PMID: 28346777
-
Conformational transition of κ-casein in micellar environment: Insight from the tryptophan fluorescence.Spectrochim Acta A Mol Biomol Spectrosc. 2017 Nov 5;186:99-104. doi: 10.1016/j.saa.2017.06.014. Epub 2017 Jun 8. Spectrochim Acta A Mol Biomol Spectrosc. 2017. PMID: 28622544
-
Synergies of Single Molecule Fluorescence and NMR for the Study of Intrinsically Disordered Proteins.Biomolecules. 2021 Dec 24;12(1):27. doi: 10.3390/biom12010027. Biomolecules. 2021. PMID: 35053175 Free PMC article. Review.
-
Protein Translational Diffusion and Intermolecular Interactions of Globular and Intrinsically Unstructured Proteins.J Phys Chem A. 2019 Nov 21;123(46):10190-10196. doi: 10.1021/acs.jpca.9b08601. Epub 2019 Nov 7. J Phys Chem A. 2019. PMID: 31657566
-
Translational diffusion of unfolded and intrinsically disordered proteins.Prog Mol Biol Transl Sci. 2019;166:85-108. doi: 10.1016/bs.pmbts.2019.05.004. Epub 2019 Jun 8. Prog Mol Biol Transl Sci. 2019. PMID: 31521238 Review.
References
-
- Kauzmann W.Some Factors in the Interpretation of Protein Denaturation. In Advances in Protein Chemistry; Anfinsen C. B., Anson M. L., Bailey K., Edsall J. T., Eds.; Academic Press, 1959; Vol. 14, pp 1–63. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

