Protein modification in neurodegenerative diseases
- PMID: 39105197
- PMCID: PMC11298556
- DOI: 10.1002/mco2.674
Protein modification in neurodegenerative diseases
Abstract
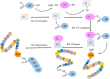
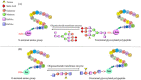
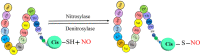
Posttranslational modifications play a crucial role in governing cellular functions and protein behavior. Researchers have implicated dysregulated posttranslational modifications in protein misfolding, which results in cytotoxicity, particularly in neurodegenerative diseases such as Alzheimer disease, Parkinson disease, and Huntington disease. These aberrant posttranslational modifications cause proteins to gather in certain parts of the brain that are linked to the development of the diseases. This leads to neuronal dysfunction and the start of neurodegenerative disease symptoms. Cognitive decline and neurological impairments commonly manifest in neurodegenerative disease patients, underscoring the urgency of comprehending the posttranslational modifications' impact on protein function for targeted therapeutic interventions. This review elucidates the critical link between neurodegenerative diseases and specific posttranslational modifications, focusing on Tau, APP, α-synuclein, Huntingtin protein, Parkin, DJ-1, and Drp1. By delineating the prominent aberrant posttranslational modifications within Alzheimer disease, Parkinson disease, and Huntington disease, the review underscores the significance of understanding the interplay among these modifications. Emphasizing 10 key abnormal posttranslational modifications, this study aims to provide a comprehensive framework for investigating neurodegenerative diseases holistically. The insights presented herein shed light on potential therapeutic avenues aimed at modulating posttranslational modifications to mitigate protein aggregation and retard neurodegenerative disease progression.
Keywords: SUMOylation; immune system; neurodegenerative diseases; posttranslational modifications.
© 2024 The Author(s). MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




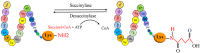
Similar articles
-
From Posttranslational Modifications to Disease Phenotype: A Substrate Selection Hypothesis in Neurodegenerative Diseases.Int J Mol Sci. 2021 Jan 18;22(2):901. doi: 10.3390/ijms22020901. Int J Mol Sci. 2021. PMID: 33477465 Free PMC article. Review.
-
Deciphering the Role of Aberrant Protein Post-Translational Modification in the Pathology of Neurodegeneration.CNS Neurol Disord Drug Targets. 2021;20(1):54-67. doi: 10.2174/1871527319666200903162200. CNS Neurol Disord Drug Targets. 2021. PMID: 32885763 Review.
-
Dynamin-related protein 1: A critical protein in the pathogenesis of neural system dysfunctions and neurodegenerative diseases.J Cell Physiol. 2019 Jul;234(7):10032-10046. doi: 10.1002/jcp.27866. Epub 2018 Dec 4. J Cell Physiol. 2019. PMID: 30515821 Review.
-
Mitochondrial biogenesis: pharmacological approaches.Curr Pharm Des. 2014;20(35):5507-9. doi: 10.2174/138161282035140911142118. Curr Pharm Des. 2014. PMID: 24606795
-
Synthetic Proteins and Peptides for the Direct Interrogation of α-Synuclein Posttranslational Modifications.Biomolecules. 2015 Jun 25;5(3):1210-27. doi: 10.3390/biom5031210. Biomolecules. 2015. PMID: 26120904 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
