The next frontier in immunotherapy: potential and challenges of CAR-macrophages
- PMID: 39103972
- PMCID: PMC11302330
- DOI: 10.1186/s40164-024-00549-9
The next frontier in immunotherapy: potential and challenges of CAR-macrophages
Abstract
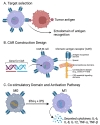
Chimeric antigen receptor macrophage (CAR-MΦ) represents a significant advancement in immunotherapy, especially for treating solid tumors where traditional CAR-T therapies face limitations. CAR-MΦ offers a promising approach to target and eradicate tumor cells by utilizing macrophages' phagocytic and antigen-presenting abilities. However, challenges such as the complex tumor microenvironment (TME), variability in antigen expression, and immune suppression limit their efficacy. This review addresses these issues, exploring mechanisms of CAR-MΦ action, optimal construct designs, and interactions within the TME. It also delves into the ex vivo manufacturing challenges of CAR-MΦ, discussing autologous and allogeneic sources and the importance of stringent quality control. The potential synergies of integrating CAR-MΦ with existing cancer therapies like checkpoint inhibitors and conventional chemotherapeutics are examined to highlight possible enhanced treatment outcomes. Furthermore, regulatory pathways for CAR-MΦ therapies are scrutinized alongside established protocols for CAR-T cells, identifying unique considerations essential for clinical trials and market approval. Proposed safety monitoring frameworks aim to manage potential adverse events, such as cytokine release syndrome, crucial for patient safety. Consolidating current research and clinical insights, this review seeks to refine CAR-MΦ therapeutic applications, overcome barriers, and suggest future research directions to transition CAR-MΦ therapies from experimental platforms to standard cancer care options.
Keywords: CAR macrophage (CAR-MΦ); Clinical trials; Combination therapies; Immunotherapy; Tumor Microenvironment (TME).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy.J Exp Clin Cancer Res. 2022 Mar 31;41(1):119. doi: 10.1186/s13046-022-02327-z. J Exp Clin Cancer Res. 2022. PMID: 35361234 Free PMC article. Review.
-
Recent Advances in CAR-Based Solid Tumor Immunotherapy.Cells. 2023 Jun 11;12(12):1606. doi: 10.3390/cells12121606. Cells. 2023. PMID: 37371075 Free PMC article. Review.
-
Beyond CAR T cells: exploring alternative cell sources for CAR-like cellular therapies.Biol Chem. 2024 May 21;405(7-8):485-515. doi: 10.1515/hsz-2023-0317. Print 2024 Jul 26. Biol Chem. 2024. PMID: 38766710 Review.
-
CAR-macrophage versus CAR-T for solid tumors: The race between a rising star and a superstar.Biomol Biomed. 2024 May 2;24(3):465-476. doi: 10.17305/bb.2023.9675. Biomol Biomed. 2024. PMID: 37877819 Free PMC article. Review.
-
Befriending the Hostile Tumor Microenvironment in CAR T-Cell Therapy.Front Immunol. 2021 Feb 10;11:618387. doi: 10.3389/fimmu.2020.618387. eCollection 2020. Front Immunol. 2021. PMID: 33643299 Free PMC article. Review.
Cited by
-
Empowering brain tumor management: chimeric antigen receptor macrophage therapy.Theranostics. 2024 Sep 3;14(14):5725-5742. doi: 10.7150/thno.98290. eCollection 2024. Theranostics. 2024. PMID: 39310093 Free PMC article. Review.

