IL-23 Priming Enhances the Neuroprotective Effects of MSC-Derived Exosomes in Treating Retinal Degeneration
- PMID: 39102262
- PMCID: PMC11309046
- DOI: 10.1167/iovs.65.10.8
IL-23 Priming Enhances the Neuroprotective Effects of MSC-Derived Exosomes in Treating Retinal Degeneration
Abstract
Purpose: Neuroinflammation is a characteristic feature of neurodegenerative diseases. Mesenchymal stem cell-derived exosomes (MSC-exo) have shown neuroprotective effects through immunoregulation, but the therapeutic efficacy remains unsatisfactory. This study aims to enhance the neuroprotective capacity of MSC-exo through IL-23 priming for treating retinal degeneration in mice.
Methods: MSC were primed with IL-23 stimulation in vitro, and subsequently, exosomes (MSC-exo and IL-23-MSC-exo) were isolated and characterized. Two retinal degenerative disease models (NaIO3-induced mice and rd10 mice) received intravitreal injections of these exosomes. The efficacy of exosomes was assessed by examining retinal structural and functional recovery. Furthermore, exosomal microRNA (miRNA) sequencing was conducted, and the effects of exosomes on the M1 and M2 microglial phenotype shift were evaluated.
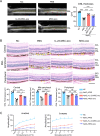
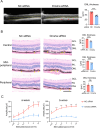
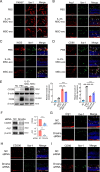
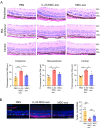
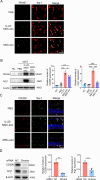
Results: IL-23-primed MSC-derived exosomes (IL-23-MSC-exo) exhibited enhanced capability in protecting photoreceptor cells and retinal pigment epithelium (RPE) cells against degenerative damage and fostering the restoration of retinal neural function in both NaIO3-induced retinal degeneration mice and rd10 mice when compared with MSC-exo. The exosomal miRNA suppression via Drosha knockdown in IL-23-primed MSC would abolish the neuroprotective role of IL-23-MSC-exo, highlighting the miRNA-dependent mechanism. Bioinformatic analysis, along with further in vivo biological studies, revealed that IL-23 priming induced a set of anti-inflammatory miRNAs in MSC-exo, prompting the transition of M1 to M2 microglial polarization.
Conclusions: IL-23 priming presents as a potential avenue for amplifying the immunomodulatory and neuroprotective effects of MSC-exo in treating retinal degeneration.
Conflict of interest statement
Disclosure:
Figures

Similar articles
-
Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization.Cardiovasc Res. 2019 Jun 1;115(7):1205-1216. doi: 10.1093/cvr/cvz040. Cardiovasc Res. 2019. PMID: 30753344 Free PMC article.
-
Exosomes from IL-1β-Primed Mesenchymal Stem Cells Inhibited IL-1β- and TNF-α-Mediated Inflammatory Responses in Osteoarthritic SW982 Cells.Tissue Eng Regen Med. 2021 Aug;18(4):525-536. doi: 10.1007/s13770-020-00324-x. Epub 2021 Jan 25. Tissue Eng Regen Med. 2021. PMID: 33495946 Free PMC article.
-
Nicorandil-Pretreated Mesenchymal Stem Cell-Derived Exosomes Facilitate Cardiac Repair After Myocardial Infarction via Promoting Macrophage M2 Polarization by Targeting miR-125a-5p/TRAF6/IRF5 Signaling Pathway.Int J Nanomedicine. 2024 Feb 29;19:2005-2024. doi: 10.2147/IJN.S441307. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38469055 Free PMC article.
-
Compounding engineered mesenchymal stem cell-derived exosomes: A potential rescue strategy for retinal degeneration.Biomed Pharmacother. 2024 Apr;173:116424. doi: 10.1016/j.biopha.2024.116424. Epub 2024 Mar 11. Biomed Pharmacother. 2024. PMID: 38471273 Review.
-
New Therapeutic Strategies for the Inflammatory Rheumatoid Arthritis Disease: Emphasizing Mesenchymal Stem Cells and Associated exo-miRNA or exo-lncRNA.Cell Biochem Biophys. 2024 Sep;82(3):1599-1611. doi: 10.1007/s12013-024-01316-7. Epub 2024 May 31. Cell Biochem Biophys. 2024. PMID: 38822204 Review.
References
-
- Jin ZB, Gao ML, Deng WL, et al. .. Stemming retinal regeneration with pluripotent stem cells. Prog Retin Eye Res. 2019; 69: 38–56. - PubMed
-
- Dhodapkar RM, Martell D, Hafler BP.. Glial-mediated neuroinflammatory mechanisms in age-related macular degeneration. Semin Immunopathol. 2022; 44: 673–683. - PubMed
-
- Karlstetter M, Scholz R, Rutar M, Wong WT, Provis JM, Langmann T.. Retinal microglia: just bystander or target for therapy? Prog Retin Eye Res. 2015; 45: 30–57. - PubMed
-
- Arnhold S, Absenger Y, Klein H, Addicks K, Schraermeyer U.. Transplantation of bone marrow-derived mesenchymal stem cells rescue photoreceptor cells in the dystrophic retina of the rhodopsin knockout mouse. Graefes Arch Clin Exp Ophthalmol. 2007; 245: 414–422. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

