Triggering of endoplasmic reticulum stress via ATF4-SPHK1 signaling promotes glioblastoma invasion and chemoresistance
- PMID: 39090107
- PMCID: PMC11294582
- DOI: 10.1038/s41419-024-06936-8
Triggering of endoplasmic reticulum stress via ATF4-SPHK1 signaling promotes glioblastoma invasion and chemoresistance
Abstract
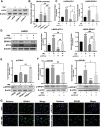
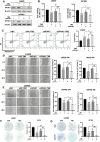
Despite advances in therapies, glioblastoma (GBM) recurrence is almost inevitable due to the aggressive growth behavior of GBM cells and drug resistance. Temozolomide (TMZ) is the preferred drug for GBM chemotherapy, however, development of TMZ resistance is over 50% cases in GBM patients. To investigate the mechanism of TMZ resistance and invasive characteristics of GBM, analysis of combined RNA-seq and ChIP-seq was performed in GBM cells in response to TMZ treatment. We found that the PERK/eIF2α/ATF4 signaling was significantly upregulated in the GBM cells with TMZ treatment, while blockage of ATF4 effectively inhibited cell migration and invasion. SPHK1 expression was transcriptionally upregulated by ATF4 in GBM cells in response to TMZ treatment. Blockage of ATF4-SPHK1 signaling attenuated the cellular and molecular events in terms of invasive characteristics and TMZ resistance. In conclusion, GBM cells acquired chemoresistance in response to TMZ treatment via constant ER stress. ATF4 transcriptionally upregulated SPHK1 expression to promote GBM cell aggression and TMZ resistance. The ATF4-SPHK1 signaling in the regulation of the transcription factors of EMT-related genes could be the underlying mechanism contributing to the invasion ability of GBM cells and TMZ resistance. ATF4-SPHK1-targeted therapy could be a potential strategy against TMZ resistance in GBM patients.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Nuclear factor I A promotes temozolomide resistance in glioblastoma via activation of nuclear factor κB pathway.Life Sci. 2019 Nov 1;236:116917. doi: 10.1016/j.lfs.2019.116917. Epub 2019 Oct 12. Life Sci. 2019. PMID: 31614149
-
MicroRNA-128-3p Enhances the Chemosensitivity of Temozolomide in Glioblastoma by Targeting c-Met and EMT.Sci Rep. 2020 Jun 11;10(1):9471. doi: 10.1038/s41598-020-65331-3. Sci Rep. 2020. PMID: 32528036 Free PMC article.
-
Exosomal transfer of miR-151a enhances chemosensitivity to temozolomide in drug-resistant glioblastoma.Cancer Lett. 2018 Nov 1;436:10-21. doi: 10.1016/j.canlet.2018.08.004. Epub 2018 Aug 10. Cancer Lett. 2018. PMID: 30102952
-
MicroRNAs as the pivotal regulators of Temozolomide resistance in glioblastoma.Mol Brain. 2024 Jul 2;17(1):42. doi: 10.1186/s13041-024-01113-6. Mol Brain. 2024. PMID: 38956588 Free PMC article. Review.
-
Harnessing the role of aberrant cell signaling pathways in glioblastoma multiforme: a prospect towards the targeted therapy.Mol Biol Rep. 2024 Oct 19;51(1):1069. doi: 10.1007/s11033-024-09996-3. Mol Biol Rep. 2024. PMID: 39424705 Review.
Cited by
-
Exploring the prognostic value and potential therapeutic strategies of MS4A6A in glioblastoma: A comprehensive analysis of single-cell and multi-omics data.J Cell Mol Med. 2024 Oct;28(20):e70177. doi: 10.1111/jcmm.70177. J Cell Mol Med. 2024. PMID: 39470579 Free PMC article.
References
MeSH terms
Substances
Grants and funding
- 2019SCZ051, 2021SCZ06, 2020SCZ09, 2023SCZ60/Department of Finance of Jilin Province (Jilin Provincial Department of Finance)
- YDZJ202201ZYTS247, 20200403079SF, YDZJ202301ZYTS035/Department of Science and Technology of Jilin Province (Jilin Province Science and Technology Department)
- 32300594/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources

