Current Treatments for Generalized Pustular Psoriasis: A Narrative Summary of a Systematic Literature Search
- PMID: 39088126
- PMCID: PMC11393368
- DOI: 10.1007/s13555-024-01230-z
Current Treatments for Generalized Pustular Psoriasis: A Narrative Summary of a Systematic Literature Search
Abstract
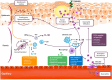
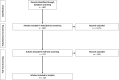
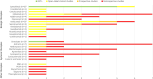
Generalized pustular psoriasis (GPP) is a rare, chronic and potentially life-threatening autoinflammatory skin disease characterized by widespread eruption of sterile pustules, with or without systemic inflammation. GPP can significantly reduce patients' quality of life (QoL). Several therapeutic approaches have been described in the literature, but there is no consensus on optimal treatment. In this review, we summarize published literature on efficacy, safety and QoL outcomes associated with current treatment of GPP with both approved and non-approved products. Embase and MEDLINE databases were searched (1980-September 2023). A search protocol was designed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and registered on the PROSPERO database (CRD42021215437). Details on publication, population, intervention, efficacy, safety and QoL were captured and checked by independent reviewers. In total, 118 publications were included, with only 19% of publications reporting on the results of clinical trials. Treatment modalities reported for GPP included non-biologic systemic therapies such as retinoids, cyclosporine and methotrexate, topical agents, biologics and small molecules, among others. Results were highly heterogeneous and methodological quality was very low, with only the interleukin-36R inhibitor spesolimab reporting results from placebo-controlled randomized trials; based on this, spesolimab is now approved for GPP treatment in regions including the USA, Japan, China, the EU and several other countries. Some other biologics are approved exclusively in Japan and Taiwan for the treatment of GPP based on open-label studies with small patient numbers in lieu of double-blind studies. Non-standardization of clinical outcomes across studies remains a major hurdle in reaching a consensus on optimal treatment. However, recently trials have been conducted using well-defined, disease-specific endpoints to evaluate GPP-targeted treatments, which will hopefully advance patient care. In conclusion, this review highlights the need for prospective randomized studies with GPP-specific endpoints to determine the optimal treatment strategy.
Keywords: Autoinflammation; GPP; Generalized pustular psoriasis; IL-36R inhibitor; Neutrophilic dermatoses; Psoriasis.
Plain language summary
Generalized pustular psoriasis (GPP) is a rare, chronic skin condition characterized by painful, sterile pustules that can occur all over the body. These pustules may also be accompanied by systemic inflammation, which can lead to serious health complications. GPP significantly impacts patients’ quality of life and can even be life-threatening. Because the disease is so rare, treatment guidelines have typically been based on those for plaque psoriasis. However, these guidelines do not specifically address the unique needs of GPP. In this review, we analysed the published literature on GPP management, focussing on treatment efficacy, safety and quality of life outcomes. We searched the literature databases Embase and MEDLINE for articles published between 1980 and September 2023. In total, we identified 118 publications on this topic, covering a wide range of therapies; only one of these therapies, spesolimab, reported results from placebo-controlled randomized trials. Based on these trials, spesolimab is now approved for GPP treatment in the USA, Japan, China, the EU and several other countries. Some other therapies are approved exclusively in Japan and Taiwan based on small, open-label studies in the absence of higher-quality data. To date, comparing treatments has been challenging because of different clinical outcomes used to measure effectiveness. However, well-defined endpoints specific to GPP have recently been developed and used in trials. In conclusion, our review highlights the need for prospective randomized studies with GPP-specific endpoints to determine the best treatment strategy.
© 2024. The Author(s).
Conflict of interest statement
Lluís Puig has received consultancy/speaker’s honoraria from and/or participated in clinical trials sponsored by AbbVie, Almirall, Amgen, Baxalta, Biogen, Boehringer Ingelheim, Celgene, Gebro Pharma, Janssen, JS BIOCAD, LEO Pharma, Lilly, Merck Serono, MSD, Mylan, Novartis, Pfizer, Regeneron, Roche, Sandoz, Samsung Bioepis, Sanofi and UCB. Hideki Fujita has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, as well as grants as an investigator from AbbVie, Amgen, Boehringer Ingelheim, Celgene, Chugai Pharmaceutical, Eisai, Eli Lilly, Janssen, Japan Blood Products Organization, JMEC, Kaken, Kyorin, Kyowa Kirin, LEO Pharma, Maruho, Mitsubishi Tanabe Pharma, Nihon Pharmaceutical, Novartis, Sanofi, Sun Pharma, Taiho, Torii, UCB and Ushio. Diamant Thaçi declares having attended advisory boards and/or received consultancy fees and/or receiving grants as an investigator from AbbVie, Almirall, Amgen, Beiersdorf, Bristol Myers Squibb, Boehringer Ingelheim, Eli Lilly, Galapagos, Janssen-Cilag, LEO Pharma, Novartis, Pfizer, Regeneron Pharmaceuticals, Inc., Samsung, Sanofi, Sun Pharmaceutical Industries and UCB. Min Zheng has received grants, consulting fees and/or speaker’s fees from AbbVie, Boehringer Ingelheim, Janssen-Cilag, LEO Pharma China, Novartis, Pfizer and Xian-Janssen. Ana Cristina Hernandez Daly is an employee of Boehringer Ingelheim. Craig Leonardi has received honoraria or fees for serving on advisory boards, as a speaker and as a consultant, as well as grants as an investigator from AbbVie, Actavis, Amgen, Boehringer Ingelheim, Celgene, Coherus, Dermira, Eli Lilly, Galderma, Janssen, Merck, Novartis, Pfizer, LEO Pharma, Sandoz, Stiefel, UCB, Vitae and Wyeth. Mark G. Lebwohl is an employee of Mount Sinai and receives research funds from: AbbVie, Amgen, Arcutis, Avotres, Boehringer Ingelheim, Cara Therapeutics, Dermavant Sciences, Eli Lilly, Incyte, Janssen Research & Development, LLC, Ortho Dermatologics, Regeneron, and UCB, Inc., and is a consultant for Aditum Bio, Almirall, AltruBio Inc., AnaptysBio, Arcutis, Inc., Arena Pharmaceuticals, Aristea Therapeutics, Avotres Therapeutics, BiomX, Brickell Biotech, Boehringer Ingelheim, Bristol Myers Squibb, Cara Therapeutics, Castle Biosciences, Celltrion, Corevitas, Dermavant Sciences, Evommune, Inc., Facilitation of International Dermatology Education, Forte Biosciences, Foundation for Research and Education in Dermatology, Hexima Ltd, Incyte, LEO Pharma, Meiji Seika Pharma, Mindera, Pfizer, Seanergy, Trevi, Vial, and Verrica. Jonathan Barker declares having attended advisory boards and/or received consultancy fees and/or spoken at sponsored symposia, and/or received grant funding from AbbVie, Almirall, Amgen, AnaptysBio, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Samsung, Sienna, Sun Pharmaceutical Industries and UCB.
Figures

Similar articles
-
Design of Effisayil™ 2: A Randomized, Double-Blind, Placebo-Controlled Study of Spesolimab in Preventing Flares in Patients with Generalized Pustular Psoriasis.Dermatol Ther (Heidelb). 2023 Jan;13(1):347-359. doi: 10.1007/s13555-022-00835-6. Epub 2022 Nov 5. Dermatol Ther (Heidelb). 2023. PMID: 36333618 Free PMC article.
-
Study protocol of the global Effisayil 1 Phase II, multicentre, randomised, double-blind, placebo-controlled trial of spesolimab in patients with generalized pustular psoriasis presenting with an acute flare.BMJ Open. 2021 Mar 30;11(3):e043666. doi: 10.1136/bmjopen-2020-043666. BMJ Open. 2021. PMID: 33785490 Free PMC article.
-
Generalized Pustular Psoriasis: A Review on Clinical Characteristics, Diagnosis, and Treatment.Dermatol Ther (Heidelb). 2023 Mar;13(3):673-688. doi: 10.1007/s13555-022-00881-0. Epub 2023 Jan 13. Dermatol Ther (Heidelb). 2023. PMID: 36635445 Free PMC article. Review.
-
Pustular Psoriasis: A Narrative Review of Recent Developments in Pathophysiology and Therapeutic Options.Dermatol Ther (Heidelb). 2021 Dec;11(6):1917-1929. doi: 10.1007/s13555-021-00612-x. Epub 2021 Oct 9. Dermatol Ther (Heidelb). 2021. PMID: 34626330 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
References
-
- Gooderham MJ, Van Voorhees AS, Lebwohl MG. An update on generalized pustular psoriasis. Expert Rev Clin Immunol. 2019;15(9):907–19. - PubMed
-
- Zelickson BD, Muller SA. Generalized pustular psoriasis. A review of 63 cases. Arch Dermatol. 1991;127(9):1339–45. - PubMed
-
- Augey F, Renaudier P, Nicolas JF. Generalized pustular psoriasis (Zumbusch): a French epidemiological survey. Eur J Dermatol. 2006;16(6):669–73. - PubMed
-
- Ohkawara A, Yasuda H, Kobayashi H, et al. Generalized pustular psoriasis in Japan: two distinct groups formed by differences in symptoms and genetic background. Acta Derm Venereol. 1996;76(1):68–71. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

