Physiological Response to the COVID-19 Vaccine: Insights From a Prospective, Randomized, Single-Blinded, Crossover Trial
- PMID: 39083770
- PMCID: PMC11325110
- DOI: 10.2196/51120
Physiological Response to the COVID-19 Vaccine: Insights From a Prospective, Randomized, Single-Blinded, Crossover Trial
Abstract
Background: Rapid development and implementation of vaccines constituted a crucial step in containing the COVID-19 pandemic. A comprehensive understanding of physiological responses to these vaccines is important to build trust in medicine.
Objective: This study aims to investigate temporal dynamics before and after COVID-19 vaccination in 4 physiological parameters as well as the duration of menstrual cycle phases.
Methods: In a prospective trial, 17,825 adults in the Netherlands wore a medical device on their wrist for up to 9 months. The device recorded their physiological signals and synchronized with a complementary smartphone app. By means of multilevel quadratic regression, we examined changes in wearable-recorded breathing rate, wrist skin temperature, heart rate, heart rate variability, and objectively assessed the duration of menstrual cycle phases in menstruating participants to assess the effects of COVID-19 vaccination.
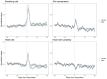
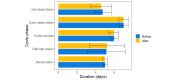
Results: The recorded physiological signals demonstrated short-term increases in breathing rate and heart rate after COVID-19 vaccination followed by a prompt rebound to baseline levels likely reflecting biological mechanisms accompanying the immune response to vaccination. No sex differences were evident in the measured physiological responses. In menstruating participants, we found a 0.8% decrease in the duration of the menstrual phase following vaccination.
Conclusions: The observed short-term changes suggest that COVID-19 vaccines are not associated with long-term biophysical issues. Taken together, our work provides valuable insights into continuous fluctuations of physiological responses to vaccination and highlights the importance of digital solutions in health care.
International registered report identifier (irrid): RR2-10.1186/s13063-021-05241-5.
Keywords: SARS-CoV-2; biological mechanism; biosignals; breathing rate; development; digital health; heart rate; immune response; implementation; medical device; menstrual cycle; sex; vaccination; vaccine; vaccine reactogenicity; wearable technology; wearables.
©Andjela Markovic, Vladimir Kovacevic, Timo B Brakenhoff, Duco Veen, Paul Klaver, Marianna Mitratza, George S Downward, Diederick E Grobbee, Maureen Cronin, Brianna M Goodale, COVID-19 Remote Early Detection (COVID-RED) consortium. Originally published in the Journal of Medical Internet Research (https://www.jmir.org), 31.07.2024.
Conflict of interest statement
Conflicts of Interest: AM, VK, MC, and BMG are past employees of Ava AG. TBB, DV, PK, and BMG are current or past employees of Julius Clinical Research. MM is a current employee of P95 CVBA. The authors have no financial or personal conflicts of interest to declare.
Figures
Similar articles
-
A prospective, randomized, single-blinded, crossover trial to investigate the effect of a wearable device in addition to a daily symptom diary for the remote early detection of SARS-CoV-2 infections (COVID-RED): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Jun 22;22(1):412. doi: 10.1186/s13063-021-05241-5. Trials. 2021. PMID: 34158099 Free PMC article.
-
A prospective, randomized, single-blinded, crossover trial to investigate the effect of a wearable device in addition to a daily symptom diary for the Remote Early Detection of SARS-CoV-2 infections (COVID-RED): a structured summary of a study protocol for a randomized controlled trial.Trials. 2021 Oct 11;22(1):694. doi: 10.1186/s13063-021-05643-5. Trials. 2021. PMID: 34635140 Free PMC article.
-
COVID-19 Vaccine Effectiveness and Digital Pandemic Surveillance in Germany (eCOV Study): Web Application-Based Prospective Observational Cohort Study.J Med Internet Res. 2024 Jun 4;26:e47070. doi: 10.2196/47070. J Med Internet Res. 2024. PMID: 38833299 Free PMC article.
-
Insights into COVID-19 Vaccine Development Based on Immunogenic Structural Proteins of SARS-CoV-2, Host Immune Responses, and Herd Immunity.Cells. 2021 Oct 29;10(11):2949. doi: 10.3390/cells10112949. Cells. 2021. PMID: 34831172 Free PMC article. Review.
-
Menstrual abnormalities after COVID-19 vaccines: A systematic review.Vacunas. 2022 Sep-Dec;23:S77-S87. doi: 10.1016/j.vacun.2022.07.001. Epub 2022 Jul 19. Vacunas. 2022. PMID: 35873308 Free PMC article. Review.
References
-
- Vaccine BNT162b2: conditions of authorisation under Regulation 174. Medicines and Healthcare Products Regulatory Agency. 2020. [2022-10-16]. https://www.gov.uk/government/publications/regulatory-approval-of-pfizer... .
-
- Menni C, Klaser K, May A, Polidori L, Capdevila J, Louca P, Sudre CH, Nguyen LH, Drew DA, Merino J, Hu C, Selvachandran S, Antonelli M, Murray B, Canas LS, Molteni E, Graham MS, Modat M, Joshi AD, Mangino M, Hammers A, Goodman AL, Chan AT, Wolf J, Steves CJ, Valdes AM, Ourselin S, Spector TD. Vaccine side-effects and SARS-CoV-2 infection after vaccination in users of the COVID symptom study app in the UK: a prospective observational study. Lancet Infect Dis. 2021;21(7):939–949. doi: 10.1016/S1473-3099(21)00224-3. https://linkinghub.elsevier.com/retrieve/pii/S1473-3099(21)00224-3 S1473-3099(21)00224-3 - DOI - PMC - PubMed
-
- Tenforde MW, Self WH, Adams K, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Exline MC, Gong MN, Mohamed A, Henning DJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Halasa N, Chappell JD, Lauring AS, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Olson SM, Kobayashi M, Verani JR, Patel MM, InfluenzaOther Viruses in the Acutely Ill (IVY) Network Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–2054. doi: 10.1001/jama.2021.19499. https://europepmc.org/abstract/MED/34734975 2786039 - DOI - PMC - PubMed
-
- Mascellino MT, Di Timoteo F, De Angelis M, Oliva A. Overview of the main anti-SARS-CoV-2 vaccines: mechanism of action, efficacy and safety. Infect Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. https://europepmc.org/abstract/MED/34511939 315727 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

