Diagnostic testing preferences can help inform future public health response efforts: Global insights from an international survey
- PMID: 39078819
- PMCID: PMC11288416
- DOI: 10.1371/journal.pgph.0003547
Diagnostic testing preferences can help inform future public health response efforts: Global insights from an international survey
Abstract
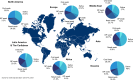
Public perception regarding diagnostic sample types as well as personal experiences can influence willingness to test. As such, public preferences for specific sample type(s) should be used to inform diagnostic and surveillance testing programs to improve public health response efforts. To understand where preferences lie, we conducted an international survey regarding the sample types used for SARS-CoV-2 tests. A Qualtrics survey regarding SARS-CoV-2 testing preferences was distributed via social media and email. The survey collected preferences regarding sample methods and key demographic data. Python was used to analyze survey responses. From March 30th to June 15th, 2022, 2,094 responses were collected from 125 countries. Participants were 55% female and predominantly aged 25-34 years (27%). Education and employment were skewed: 51% had graduate degrees, 26% had bachelor's degrees, 27% were scientists/researchers, and 29% were healthcare workers. By rank sum analysis, the most preferred sample type globally was the oral swab, followed by saliva, with parents/guardians preferring saliva-based testing for children. Respondents indicated a higher degree of trust in PCR testing (84%) vs. rapid antigen testing (36%). Preferences for self- or healthcare worker-collected sampling varied across regions. This international survey identified a preference for oral swabs and saliva when testing for SARS-CoV-2. Notably, respondents indicated that if they could be assured that all sample types performed equally, then saliva was preferred. Overall, survey responses reflected the region-specific testing experiences during the COVID-19. Public preferences should be considered when designing future response efforts to increase utilization, with oral sample types (either swabs or saliva) providing a practical option for large-scale, accessible diagnostic testing.
Copyright: © 2024 Salzano et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: ALW has received consulting and/or advisory board fees from Pfizer, Merck, Diasorin, PPS Health, Co-Diagnostics, and Global Diagnostic Systems for work unrelated to this project, and is Principal Investigator on research grants from Pfizer, Merck, NIH RADx UP and SalivaDirect, Inc. to Yale University and from NIH RADx, Balvi.io and Shield T3 to SalivaDirect, Inc. All other co-authors declare no potential conflict of interest.
Figures
Similar articles
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Willingness to Use Home Collection Methods to Provide Specimens for SARS-CoV-2/COVID-19 Research: Survey Study.J Med Internet Res. 2020 Sep 3;22(9):e19471. doi: 10.2196/19471. J Med Internet Res. 2020. PMID: 32790639 Free PMC article.
-
Surveillance testing using salivary RT-PCR for SARS-CoV-2 in managed quarantine facilities in Australia: A laboratory validation and implementation study.Lancet Reg Health West Pac. 2022 Sep;26:100533. doi: 10.1016/j.lanwpc.2022.100533. Epub 2022 Jul 8. Lancet Reg Health West Pac. 2022. PMID: 35821908 Free PMC article.
-
Comparing Preferences for Depression and Diabetes Treatment among Adults of Different Racial and Ethnic Groups Who Reported Discrimination in Health Care [Internet].Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2021 Jan. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI); 2021 Jan. PMID: 38478703 Free Books & Documents. Review.
-
Saliva as a sample type for SARS-CoV-2 detection: implementation successes and opportunities around the globe.Expert Rev Mol Diagn. 2022 May;22(5):519-535. doi: 10.1080/14737159.2022.2094250. Epub 2022 Jul 4. Expert Rev Mol Diagn. 2022. PMID: 35763281 Review.
References
-
- Gagnon F, Bhatt M, Zemek R, Webster RJ, Johnson-Obaseki S, Harman S. Nasopharyngeal swabs vs. saliva sampling for SARS-CoV-2 detection: A cross-sectional survey of acceptability for caregivers and children after experiencing both methods. PLoS One. 2022;17: e0270929. doi: 10.1371/journal.pone.0270929 - DOI - PMC - PubMed
-
- Van Rossum G, Drake FL. Python 3 Reference Manual. Scotts Valley, CA: CreateSpace; 2009.
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous