Cytokines in PD-1 immune checkpoint inhibitor adverse events and implications for the treatment of uveitis
- PMID: 39075390
- PMCID: PMC11285394
- DOI: 10.1186/s12886-024-03575-7
Cytokines in PD-1 immune checkpoint inhibitor adverse events and implications for the treatment of uveitis
Abstract
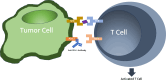
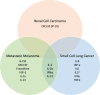
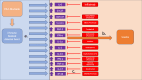
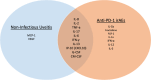
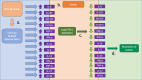
Immune checkpoint inhibitors (ICI) such as Programmed cell Death 1 (PD-1) inhibitors have improved cancer treatment by enhancing the immune system's ability to target malignant cells. Their use is associated with immune-related adverse events (irAEs), including uveitis. The profile of pro-inflammatory cytokines underlying Anti-PD-1-induced uveitis shares significant overlap with that of non-infectious uveitis. Current corticosteroid treatments for uveitis while effective are fraught with vision threatening side effects. The cytokine profile in ICI-related uveitis has a large overlap with that of noninfectious uveitis, this overlap strongly supports the potential for therapy that activates the PD-1 axis in the eye to treat uveitis. Indeed, ICI related uveitis often resolves with cessation of the ICI, restoring the endogenous PD-1 axis. The potential benefit of targeting many pro-inflammatory cytokines via local PD-1 axis activation is mitigating ocular inflammation while minimizing adverse effects.
Keywords: Drug Therapy; Immune Checkpoint Inhibitors; Uveitis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Evolving spectrum of drug-induced uveitis at the era of immune checkpoint inhibitors results from the WHO's pharmacovigilance database.J Autoimmun. 2020 Jul;111:102454. doi: 10.1016/j.jaut.2020.102454. Epub 2020 Apr 14. J Autoimmun. 2020. PMID: 32303423
-
Uveitis in Patients Treated with CTLA-4 and PD-1 Checkpoint Blockade Inhibition.Ocul Immunol Inflamm. 2020;28(2):217-227. doi: 10.1080/09273948.2019.1577978. Epub 2019 Mar 1. Ocul Immunol Inflamm. 2020. PMID: 30821569 Free PMC article. Review.
-
Development of Vogt-Koyanagi-Harada disease-like uveitis during treatment by anti-programmed death-1 antibody: a case report.BMC Ophthalmol. 2024 Jun 7;24(1):240. doi: 10.1186/s12886-024-03484-9. BMC Ophthalmol. 2024. PMID: 38849786 Free PMC article.
-
Ocular Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors in Lung Cancer.Front Immunol. 2021 Aug 24;12:701951. doi: 10.3389/fimmu.2021.701951. eCollection 2021. Front Immunol. 2021. PMID: 34504488 Free PMC article. Review.
-
Ophthalmic Immune-Related Adverse Events after Anti-CTLA-4 or PD-1 Therapy Recorded in the American Academy of Ophthalmology Intelligent Research in Sight Registry.Ophthalmology. 2021 Jun;128(6):910-919. doi: 10.1016/j.ophtha.2020.11.001. Epub 2020 Nov 6. Ophthalmology. 2021. PMID: 33166553
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

