Synthesis, characterization and biological research of novel 2-(quinoline-4-carbonyl)hydrazide-acrylamide hybrids as potential anticancer agents on MCF-7 breast carcinoma cells by targeting EGFR-TK
- PMID: 39071480
- PMCID: PMC11273260
- DOI: 10.1039/d4ra03963g
Synthesis, characterization and biological research of novel 2-(quinoline-4-carbonyl)hydrazide-acrylamide hybrids as potential anticancer agents on MCF-7 breast carcinoma cells by targeting EGFR-TK
Abstract
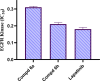
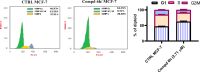
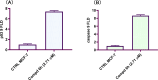
Novel derivatives of the 2-(quinoline-4-carbonyl)hydrazide scaffold carrying the acrylamide moiety were synthesized and tested for their cytotoxic efficacy against the breast carcinoma MCF-7 cell line. The most active members 6a, 6b and 6h revealed significant antiproliferative action with an IC50 value of 3.39, 5.94 and 2.71 μM, respectively, which were more potent than the reference drug Dox (IC50 = 6.18 μM). Aiming to enlighten the antiproliferative activity, compounds 6a and 6h were examined for their inhibitory potential against EGFR kinase. The results demonstrated that compound 6h displayed potent inhibitory activity, as concluded from the IC50 value (IC50 = 0.22 μM) compared to the standard drug Lapatinib (IC50 value of 0.18 μM). Compound 6h was found to induce significant cellular cycle arrest at the G1 phase and provoke apoptosis. Besides, compound 6h triggered apoptosis via upregulating p53 and initiator caspase 9 by 7.4- and 8.7-fold, respectively, compared to DMSO controls.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures


Similar articles
-
Design, synthesis, and antiproliferative screening of new quinoline derivatives bearing a cis-vinyl triamide motif as apoptosis activators and EGFR-TK inhibitors.RSC Adv. 2024 Aug 7;14(34):24781-24790. doi: 10.1039/d4ra04915b. eCollection 2024 Aug 5. RSC Adv. 2024. PMID: 39114435 Free PMC article.
-
Novel pyrazole-based COX-2 inhibitors as potential anticancer agents: Design, synthesis, cytotoxic effect against resistant cancer cells, cell cycle arrest, apoptosis induction and dual EGFR/Topo-1 inhibition.Bioorg Chem. 2023 Feb;131:106273. doi: 10.1016/j.bioorg.2022.106273. Epub 2022 Nov 14. Bioorg Chem. 2023. PMID: 36444790
-
New thieno[3,2-d]pyrimidine-based derivatives: Design, synthesis and biological evaluation as antiproliferative agents, EGFR and ARO inhibitors inducing apoptosis in breast cancer cells.Bioorg Chem. 2021 Oct;115:105208. doi: 10.1016/j.bioorg.2021.105208. Epub 2021 Jul 26. Bioorg Chem. 2021. PMID: 34365057
-
Design, synthesis and cytotoxic activity of molecular hybrids based on quinolin-8-yloxy and cinnamide hybrids and their apoptosis inducing property.RSC Adv. 2024 Apr 9;14(16):11443-11451. doi: 10.1039/d4ra01911c. eCollection 2024 Apr 3. RSC Adv. 2024. PMID: 38595714 Free PMC article.
-
Pyrazolo[3,4-d]pyrimidine-based dual EGFR T790M/HER2 inhibitors: Design, synthesis, structure-activity relationship and biological activity as potential antitumor and anticonvulsant agents.Eur J Med Chem. 2021 Mar 15;214:113222. doi: 10.1016/j.ejmech.2021.113222. Epub 2021 Jan 26. Eur J Med Chem. 2021. PMID: 33545637
Cited by
-
Design and synthesis of novel 2-(2-(4-bromophenyl)quinolin-4-yl)-1,3,4-oxadiazole derivatives as anticancer and antimicrobial candidates: in vitro and in silico studies.RSC Adv. 2024 Oct 25;14(46):34005-34026. doi: 10.1039/d4ra06712f. eCollection 2024 Oct 23. RSC Adv. 2024. PMID: 39463483 Free PMC article.
References
-
- Ebrahimi N. Manavi M. S. Faghihkhorasani F. Fakhr S. S. Baei F. J. Khorasani F. F. Zare M. M. Far N. P. Rezaei-Tazangi F. Ren J. Reiter R. J. Nabavi N. Aref A. R. Chen C. Ertas Y. N. Lu Q. Harnessing function of EMT in cancer drug resistance: a metastasis regulator determines chemotherapy response. Cancer Metastasis Rev. 2024;43:457–479. doi: 10.1007/s10555-023-10162-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

