Role of copper and SOD3-mediated extracellular redox regulation in tumor progression
- PMID: 39070539
- PMCID: PMC11273271
- DOI: 10.3164/jcbn.24-14
Role of copper and SOD3-mediated extracellular redox regulation in tumor progression
Abstract
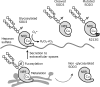
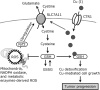
Copper (Cu), an essential micronutrient, participates in several physiological processes, including cell proliferation and development. Notably, the disturbance of Cu homeostasis promotes tumor progression through the generation of oxidative stress. Chronic or excessive accumulation of reactive oxygen species (ROS) causes lipid peroxidation, protein denaturation, and enzyme inactivation, which leads to a breakdown of intracellular homeostasis and exacerbates tumor progression. The disruption of the ROS scavenging mechanism also reduces resistance to oxidative stress, leading to further deterioration in a disease state, and maintenance of redox homeostasis is thought to inhibit the onset and progression of various diseases. Superoxide dismutase 3 (SOD3), a Cu-containing secretory antioxidative enzyme, plays a key role in extracellular redox regulation, and the significant reduction in SOD3 facilitates tumor progression. Furthermore, the significant induction of SOD3 participates in tumor metastasis. This review focuses on the role of Cu homeostasis and antioxidative enzymes, including SOD3, in tumor progression, to help clarify the role of redox regulation.
Keywords: copper; glutathione; reactive oxygen species; superoxide dismutase 3; tumor.
Copyright © 2024 JCBN.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
Similar articles
-
[Regulation of Extracellular Redox Homeostasis in Tumor Microenvironment].Yakugaku Zasshi. 2019;139(9):1139-1144. doi: 10.1248/yakushi.19-00128. Yakugaku Zasshi. 2019. PMID: 31474628 Review. Japanese.
-
Copper in the tumor microenvironment and tumor metastasis.J Clin Biochem Nutr. 2022 Jul;71(1):22-28. doi: 10.3164/jcbn.22-9. Epub 2022 May 13. J Clin Biochem Nutr. 2022. PMID: 35903604 Free PMC article.
-
Copper chaperone antioxidant-1, Atox-1, is involved in the induction of SOD3 in THP-1 cells.Biometals. 2018 Feb;31(1):61-68. doi: 10.1007/s10534-017-0067-1. Epub 2017 Nov 22. Biometals. 2018. PMID: 29168020
-
Caveolin-1 stabilizes ATP7A, a copper transporter for extracellular SOD, in vascular tissue to maintain endothelial function.Am J Physiol Cell Physiol. 2020 Nov 1;319(5):C933-C944. doi: 10.1152/ajpcell.00151.2020. Epub 2020 Sep 16. Am J Physiol Cell Physiol. 2020. PMID: 32936699 Free PMC article.
-
Extracellular superoxide dismutase and its role in cancer.Free Radic Biol Med. 2017 Nov;112:464-479. doi: 10.1016/j.freeradbiomed.2017.08.013. Epub 2017 Aug 24. Free Radic Biol Med. 2017. PMID: 28842347 Free PMC article. Review.
References
-
- Akaike T, Nishida M, Fujii S. Regulation of redox signalling by an electrophilic cyclic nucleotide. J Biochem 2013; 153: 131–138. - PubMed
-
- Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science 2006; 312: 1882–1883. - PubMed
-
- D'Autréaux B, Toledano MB. ROS as signalling molecules: mechanisms that generate specificity in ROS homeostasis. Nat Rev Mol Cell Biol 2007; 8: 813–824. - PubMed
-
- Kamata H, Hirata H. Redox regulation of cellular signalling. Cell Signal 1999; 11: 1–14. - PubMed
-
- Faraci FM. Vascular protection. Stroke 2003; 34: 327–329. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

