Quantitative Analysis of Glutathione and Carnosine Adducts with 4-Hydroxy-2-nonenal in Muscle in a hSOD1G93A ALS Rat Model
- PMID: 39066735
- PMCID: PMC11337210
- DOI: 10.1021/acs.chemrestox.4c00052
Quantitative Analysis of Glutathione and Carnosine Adducts with 4-Hydroxy-2-nonenal in Muscle in a hSOD1G93A ALS Rat Model
Abstract
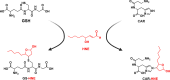
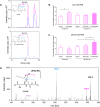
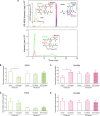
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease characterized by the dysfunction and death of motor neurons through multifactorial mechanisms that remain unclear. ALS has been recognized as a multisystemic disease, and the potential role of skeletal muscle in disease progression has been investigated. Reactive aldehydes formed as secondary lipid peroxidation products in the redox processes react with biomolecules, such as DNA, proteins, and amino acids, resulting in cytotoxic effects. 4-Hydroxy-2-nonenal (HNE) levels are elevated in the spinal cord motor neurons of ALS patients, and HNE-modified proteins have been identified in the spinal cord tissue of an ALS transgenic mice model, suggesting that reactive aldehydes can contribute to motor neuron degeneration in ALS. One biological pathway of aldehyde detoxification involves conjugation with glutathione (GSH) or carnosine (Car). Here, the detection and quantification of Car, GSH, GSSG (glutathione disulfide), and the corresponding adducts with HNE, Car-HNE, and GS-HNE, were performed in muscle and liver tissues of a hSOD1G93A ALS rat model by reverse-phase high-performance liquid chromatography coupled to electrospray ion trap tandem mass spectrometry in the selected reaction monitoring mode. A significant increase in the levels of GS-HNE and Car-HNE was observed in the muscle tissue of the end-stage ALS animals. Therefore, analyzing variations in the levels of these adducts in ALS animal tissue is crucial from a toxicological perspective and can contribute to the development of new therapeutic strategies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Similar articles
-
Detoxification of cytotoxic alpha,beta-unsaturated aldehydes by carnosine: characterization of conjugated adducts by electrospray ionization tandem mass spectrometry and detection by liquid chromatography/mass spectrometry in rat skeletal muscle.J Mass Spectrom. 2002 Dec;37(12):1219-28. doi: 10.1002/jms.381. J Mass Spectrom. 2002. PMID: 12489081
-
Proteomic analysis of 4-hydroxy-2-nonenal-modified proteins in G93A-SOD1 transgenic mice--a model of familial amyotrophic lateral sclerosis.Free Radic Biol Med. 2005 Apr 1;38(7):960-8. doi: 10.1016/j.freeradbiomed.2004.12.021. Free Radic Biol Med. 2005. PMID: 15749392
-
Aberrant DNA and RNA Methylation Occur in Spinal Cord and Skeletal Muscle of Human SOD1 Mouse Models of ALS and in Human ALS: Targeting DNA Methylation Is Therapeutic.Cells. 2022 Oct 31;11(21):3448. doi: 10.3390/cells11213448. Cells. 2022. PMID: 36359844 Free PMC article.
-
LC-ESI-MS/MS determination of 4-hydroxy-trans-2-nonenal Michael adducts with cysteine and histidine-containing peptides as early markers of oxidative stress in excitable tissues.J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Nov 15;827(1):109-18. doi: 10.1016/j.jchromb.2005.04.025. J Chromatogr B Analyt Technol Biomed Life Sci. 2005. PMID: 15916929
-
Role of 4-hydroxy-2-nonenal (HNE) in the pathogenesis of alzheimer disease and other selected age-related neurodegenerative disorders.Free Radic Biol Med. 2017 Oct;111:253-261. doi: 10.1016/j.freeradbiomed.2016.10.490. Epub 2016 Oct 24. Free Radic Biol Med. 2017. PMID: 27789292 Review.
References
-
- Hardiman O.; Al-chalabi A.; Chio A.; Corr E. M.; Logroscino G.; Robberecht W.; Shaw P. J.; Simmons Z.; van den Berg L. H. Amyotrophic Lateral Sclerosis. Nat. Rev. Dis. Primers 2017, 3, 71.10.1038/nrdp.2017.71. - DOI
-
- Rosen D. R.; Siddique T.; Patterson D.; Figlewicz D. A.; Sapp P. II; Hentati A.; Donaldson D.; Goto J.; O’Regan J. P.; Deng H.; Rahmani Z.; Krizus A.; Mckenna-yasek D.; Cayabyab A.; Gaston S. M.; Berger R.; Tanzi R. E.; Halperin J. J.; Herzfeldt B.; Van den Bergh R.; Hung W.; Bird T.; Deng G.; Mulder D. W.; Smyth C.; Laing N. G.; Soriano E.; Pericak-Vance M. A.; Haines J.; Rouleau G. A.; Gusella J. S.; Horvitz H. R.; Brown Jr R. H. Mutations in Cu/Zn Superoxide Dismutase Gene Are Associated with Familial Amyptrophic Lateral Sclerosis. Nature 1993, 362, 59–62. 10.1038/362059a0. - DOI - PubMed
-
- Borchelt D. R.; Lee M. K.; Slunt H. S.; Guarnieri M.; Xu Z.; Wong P. C.; Brown R. H.; Price D. L.; Sisodia S. S.; Cleveland D. O. N. W. Superoxide Dismutase 1 with Mutations Linked to Familial Amyotrophic Lateral Sclerosis Possesses Significant Activity. Proc. Natl. Acad. Sci. U. S. A. 1994, 91, 8292–8296. 10.1073/pnas.91.17.8292. - DOI - PMC - PubMed
-
- Rabizadeh S.; Gralla E. B.; Borchelt D. R.; Gwinn R.; Valentine J. S.; Sisodia S.; Wong P.; Lee M.; Hahn H.; Bredesen D. E. Mutations Associated with Amyotrophic Lateral Sclerosis Convert Superoxide Dismutase from an Antiapoptotic Gene to a Proapoptotic Gene: Studies in Yeast and Neural Cells. Proc. Natl. Acad. Sci. U. S. A. 1995, 92, 3024–3028. 10.1073/pnas.92.7.3024. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

