Gut Dysbiosis in the First-Passed Meconium Microbiomes of Korean Preterm Infants Compared to Full-Term Neonates
- PMID: 39065040
- PMCID: PMC11279035
- DOI: 10.3390/microorganisms12071271
Gut Dysbiosis in the First-Passed Meconium Microbiomes of Korean Preterm Infants Compared to Full-Term Neonates
Abstract
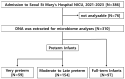
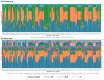
Since gestational age (GA) is an important factor influencing the presence of specific microbiomes, we aimed to characterize the core microbiomes of preterm infants compared to full-term (FT) infants. This study investigated the differences in microbiota composition between very preterm (VP), moderate-to-late preterm (MLP), and FT neonates by examining the core microbiomes of a large cohort of Korean neonates. Meconium samples from 310 neonates with a GA range of 22-40 weeks were collected, and 16S rRNA analyses were performed; 97 samples were obtained from the FT, 59 from the VP, and 154 from the MLP group. Firmicutes, Bacteroidetes, and Proteobacteria were the phylum-level core microbiomes. Infants born before 37 weeks showed a disruption in the core microbiomes. At the phylum level, the relative abundance of Bacteroidetes was positively (r = 0.177, p = 0.002) correlated with GA, while that of Proteobacteria was negatively (r = -0.116, p = 0.040) correlated with GA. At the genus level, the relative abundances of Bacteroides and Prevotella were positively correlated with GA (r = 0.157, p = 0.006; r = 0.160, p = 0.005). The meconium of preterm infants exhibited significantly lower α-diversities than that of FT infants. β-diversities did not appear to differ between the groups. Overall, these findings underscore the importance of GA in shaping the early gut microbiome.
Keywords: dysbiosis; meconium; microbiome; very preterm.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



Similar articles
-
Compositional Differences of Meconium Microbiomes of Preterm and Term Infants, and Infants That Developed Necrotizing Enterocolitis or Feeding Intolerance.Pathogens. 2022 Dec 29;12(1):55. doi: 10.3390/pathogens12010055. Pathogens. 2022. PMID: 36678403 Free PMC article.
-
Early gut microbiota in very low and extremely low birth weight preterm infants with feeding intolerance: a prospective case-control study.J Microbiol. 2022 Oct;60(10):1021-1031. doi: 10.1007/s12275-022-2180-2. Epub 2022 Aug 19. J Microbiol. 2022. PMID: 35984614 Free PMC article.
-
Perinatal Antibiotic Exposure Affects the Transmission between Maternal and Neonatal Microbiota and Is Associated with Early-Onset Sepsis.mSphere. 2020 Feb 19;5(1):e00984-19. doi: 10.1128/mSphere.00984-19. mSphere. 2020. PMID: 32075882 Free PMC article.
-
Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis.Microbiome. 2017 Mar 9;5(1):31. doi: 10.1186/s40168-017-0248-8. Microbiome. 2017. PMID: 28274256 Free PMC article. Review.
-
Exploring the Role of Gut Bacteria in Health and Disease in Preterm Neonates.Int J Environ Res Public Health. 2020 Sep 23;17(19):6963. doi: 10.3390/ijerph17196963. Int J Environ Res Public Health. 2020. PMID: 32977611 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources

