Variations of VEGFR2 Chemical Space: Stimulator and Inhibitory Peptides
- PMID: 39063029
- PMCID: PMC11276785
- DOI: 10.3390/ijms25147787
Variations of VEGFR2 Chemical Space: Stimulator and Inhibitory Peptides
Abstract
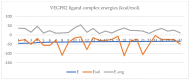
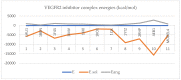
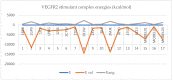
The kinase pathway plays a crucial role in blood vessel function. Particular attention is paid to VEGFR type 2 angiogenesis and vascular morphogenesis as the tyrosine kinase pathway is preferentially activated. In silico studies were performed on several peptides that affect VEGFR2 in both stimulating and inhibitory ways. This investigation aims to examine the molecular properties of VEGFR2, a molecule primarily involved in the processes of vasculogenesis and angiogenesis. These relationships were defined by the interactions between Vascular Endothelial Growth Factor receptor 2 (VEGFR2) and the structural features of the systems. The chemical space of the inhibitory peptides and stimulators was described using topological and energetic properties. Furthermore, chimeric models of stimulating and inhibitory proteins (for VEGFR2) were computed using the protein system structures. The interaction between the chimeric proteins and VEGFR was computed. The chemical space was further characterized using complex manifolds and high-dimensional data visualization. The results show that a slightly similar chemical area is shared by VEGFR2 and stimulating and inhibitory proteins. On the other hand, the stimulator peptides and the inhibitors have distinct chemical spaces.
Keywords: angiogenesis; chimeric protein; docking; molecular modeling; peripheral artery disease; vascular morphogenesis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
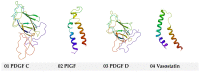



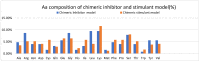


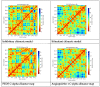
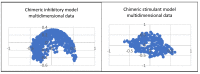

Similar articles
-
Molecular Motifs in Vascular Morphogenesis: Vascular Endothelial Growth Factor A (VEGFA) as the Leading Promoter of Angiogenesis.Int J Mol Sci. 2023 Jul 29;24(15):12169. doi: 10.3390/ijms241512169. Int J Mol Sci. 2023. PMID: 37569543 Free PMC article.
-
Substrate-specific conformational regulation of the receptor tyrosine kinase VEGFR2 catalytic domain.ACS Chem Biol. 2013 May 17;8(5):978-86. doi: 10.1021/cb400040z. Epub 2013 Mar 6. ACS Chem Biol. 2013. PMID: 23441851
-
Molecular dynamics guided insight, binding free energy calculations and pharmacophore-based virtual screening for the identification of potential VEGFR2 inhibitors.J Recept Signal Transduct Res. 2019 Oct-Dec;39(5-6):415-433. doi: 10.1080/10799893.2019.1690509. Epub 2019 Nov 22. J Recept Signal Transduct Res. 2019. PMID: 31755336
-
Structural and molecular insights from dual inhibitors of EGFR and VEGFR2 as a strategy to improve the efficacy of cancer therapy.Chem Biol Drug Des. 2024 May;103(5):e14534. doi: 10.1111/cbdd.14534. Chem Biol Drug Des. 2024. PMID: 38697951 Review.
-
Development and strategies of VEGFR-2/KDR inhibitors.Future Med Chem. 2012 Sep;4(14):1839-52. doi: 10.4155/fmc.12.121. Future Med Chem. 2012. PMID: 23043480 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

