The Role and Therapeutic Potential of Pyroptosis in Colorectal Cancer: A Review
- PMID: 39062587
- PMCID: PMC11274949
- DOI: 10.3390/biom14070874
The Role and Therapeutic Potential of Pyroptosis in Colorectal Cancer: A Review
Abstract
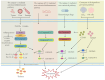
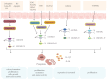
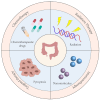
Colorectal cancer (CRC) is one of the leading causes of cancer-related mortality worldwide. The unlimited proliferation of tumor cells is one of the key features resulting in the malignant development and progression of CRC. Consequently, understanding the potential proliferation and growth molecular mechanisms and developing effective therapeutic strategies have become key in CRC treatment. Pyroptosis is an emerging type of regulated cell death (RCD) that has a significant role in cells proliferation and growth. For the last few years, numerous studies have indicated a close correlation between pyroptosis and the occurrence, progression, and treatment of many malignancies, including CRC. The development of effective therapeutic strategies to inhibit tumor growth and proliferation has become a key area in CRC treatment. Thus, this review mainly summarized the different pyroptosis pathways and mechanisms, the anti-tumor (tumor suppressor) and protective roles of pyroptosis in CRC, and the clinical and prognostic value of pyroptosis in CRC, which may contribute to exploring new therapeutic strategies for CRC.
Keywords: colorectal cancer; growth; proliferation; pyroptosis; therapy.
Conflict of interest statement
There are no conflicts of interest and disclosures associated with the manuscript.
Figures
Similar articles
-
A438079 affects colorectal cancer cell proliferation, migration, apoptosis, and pyroptosis by inhibiting the P2X7 receptor.Biochem Biophys Res Commun. 2021 Jun 18;558:147-153. doi: 10.1016/j.bbrc.2021.04.076. Epub 2021 Apr 26. Biochem Biophys Res Commun. 2021. PMID: 33915328
-
Pyroptosis is involved in the inhibitory effect of FL118 on growth and metastasis in colorectal cancer.Life Sci. 2020 Sep 15;257:118065. doi: 10.1016/j.lfs.2020.118065. Epub 2020 Jul 11. Life Sci. 2020. PMID: 32659366
-
Therapeutic inhibition of SGK1 suppresses colorectal cancer.Exp Mol Med. 2017 Nov 24;49(11):e399. doi: 10.1038/emm.2017.184. Exp Mol Med. 2017. PMID: 29170478 Free PMC article.
-
Therapeutic Potential of Polyphenols and their Nanoformulations in the Treatment of Colorectal Cancer.Anticancer Agents Med Chem. 2021 Oct 28;21(16):2117-2129. doi: 10.2174/1871520621666201231144007. Anticancer Agents Med Chem. 2021. PMID: 33390126 Review.
-
Targeted regulated cell death with small molecule compounds in colorectal cancer: Current perspectives of targeted therapy and molecular mechanisms.Eur J Med Chem. 2024 Feb 5;265:116040. doi: 10.1016/j.ejmech.2023.116040. Epub 2023 Dec 12. Eur J Med Chem. 2024. PMID: 38142509 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- 2023JJ60368/Natural Science Foundation of Hunan Province
- 32170726/National Natural Science Foundation of China
- 2022JJ30538/Natural Science Foundation of Hunan Province
- 20201919/Scientific Research Fund Project of Hunan Provincial Health Commission
- 202104010105/Scientific Research Fund Project of Hunan Provincial Health Commission
LinkOut - more resources
Full Text Sources
Medical

