Optimizing Ex Vivo CAR-T Cell-Mediated Cytotoxicity Assay through Multimodality Imaging
- PMID: 39061136
- PMCID: PMC11274748
- DOI: 10.3390/cancers16142497
Optimizing Ex Vivo CAR-T Cell-Mediated Cytotoxicity Assay through Multimodality Imaging
Abstract
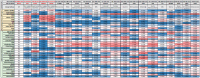
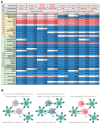
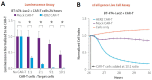
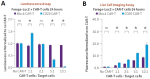
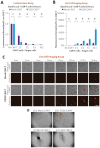
CAR-T cell-based therapies have demonstrated remarkable efficacy in treating malignant cancers, especially liquid tumors, and are increasingly being evaluated in clinical trials for solid tumors. With the FDA's initiative to advance alternative methods for drug discovery and development, full human ex vivo assays are increasingly essential for precision CAR-T development. However, prevailing ex vivo CAR-T cell-mediated cytotoxicity assays are limited by their use of radioactive materials, lack of real-time measurement, low throughput, and inability to automate, among others. To address these limitations, we optimized the assay using multimodality imaging methods, including bioluminescence, impedance tracking, phase contrast, and fluorescence, to track CAR-T cells co-cultured with CD19, CD20, and HER2 luciferase reporter cancer cells in real-time. Additionally, we varied the ratio of CAR-T cells to cancer cells to determine optimal cytotoxicity readouts. Our findings demonstrated that the CAR-T cell group effectively attacked cancer cells, and the optimized assay provided superior temporal and spatial precision measurements of ex vivo CAR-T killing of cancer cells, confirming the reliability, consistency, and high throughput of the optimized assay.
Keywords: CAR-T; CD19; CD20; HER2; cytotoxicity assay; multimodality imaging.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Development of optimized cytotoxicity assays for assessing the antitumor potential of CAR-T cells.J Immunol Methods. 2024 Feb;525:113603. doi: 10.1016/j.jim.2023.113603. Epub 2023 Dec 24. J Immunol Methods. 2024. PMID: 38147898
-
A tandem CD19/CD20 CAR lentiviral vector drives on-target and off-target antigen modulation in leukemia cell lines.J Immunother Cancer. 2017 May 16;5:42. doi: 10.1186/s40425-017-0246-1. eCollection 2017. J Immunother Cancer. 2017. PMID: 28515942 Free PMC article.
-
Visualizing CAR-T cell Immunotherapy Using 3 Tesla Fluorine-19 MRI.Mol Imaging Biol. 2022 Apr;24(2):298-308. doi: 10.1007/s11307-021-01672-3. Epub 2021 Nov 16. Mol Imaging Biol. 2022. PMID: 34786668 Free PMC article.
-
Development of a compact bidirectional promoter-driven dual chimeric antigen receptor (CAR) construct targeting CD19 and CD20 in the Sleeping Beauty (SB) transposon system.J Immunother Cancer. 2024 Apr 27;12(4):e008555. doi: 10.1136/jitc-2023-008555. J Immunother Cancer. 2024. PMID: 38677881 Free PMC article.
-
Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration.Eur J Cancer. 2022 Jan;160:112-133. doi: 10.1016/j.ejca.2021.10.016. Epub 2021 Nov 25. Eur J Cancer. 2022. PMID: 34840026 Review.
References
-
- Sadelain M., Brentjens R., Riviere I. The basic principles of chimeric antigen receptor design. Cancer Discov. 2013;3:388–398. doi: 10.1158/2159-8290.CD-12-0548. - DOI - PMC - PubMed
-
- Kochenderfer J.N., Wilson W.H., Janik J.E., Dudley M.E., Stetler-Stevenson M., Feldman S.A., Maric I., Raffeld M., Nathan D.A., Lanier B.J., et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood. 2010;116:4099–4102. doi: 10.1182/blood-2010-04-281931. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

