Recombinant Ixodes scapularis Calreticulin Binds Complement Proteins but Does Not Protect Borrelia burgdorferi from Complement Killing
- PMID: 39057787
- PMCID: PMC11280304
- DOI: 10.3390/pathogens13070560
Recombinant Ixodes scapularis Calreticulin Binds Complement Proteins but Does Not Protect Borrelia burgdorferi from Complement Killing
Abstract
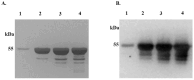
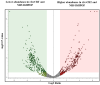
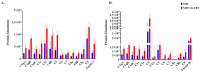
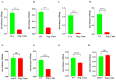
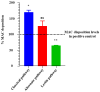
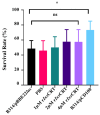
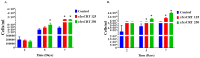
Ixodes scapularis is a blood-feeding obligate ectoparasite responsible for transmitting the Lyme disease (LD) agent, Borrelia burgdorferi. During the feeding process, I. scapularis injects B. burgdorferi into the host along with its saliva, facilitating the transmission and colonization of the LD agent. Tick calreticulin (CRT) is one of the earliest tick saliva proteins identified and is currently utilized as a biomarker for tick bites. Our recent findings revealed elevated levels of CRT in the saliva proteome of B. burgdorferi-infected I. scapularis nymphs compared to uninfected ticks. Differential precipitation of proteins (DiffPOP) and LC-MS/MS analyses were used to identify the interactions between Ixs (I. scapularis) CRT and human plasma proteins and further explore its potential role in shielding B. burgdorferi from complement killing. We observed that although yeast-expressed recombinant (r) IxsCRT binds to the C1 complex (C1q, C1r, and C1s), the activator of complement via the classical cascade, it did not inhibit the deposition of the membrane attack complex (MAC) via the classical pathway. Intriguingly, rIxsCRT binds intermediate complement proteins (C3, C5, and C9) and reduces MAC deposition through the lectin pathway. Despite the inhibition of MAC deposition in the lectin pathway, rIxsCRT did not protect a serum-sensitive B. burgdorferi strain (B314/pBBE22Luc) from complement-induced killing. As B. burgdorferi establishes a local dermal infection before disseminating to secondary organs, it is noteworthy that rIxsCRT promotes the replication of B. burgdorferi in culture. We hypothesize that rIxsCRT may contribute to the transmission and/or host colonization of B. burgdorferi by acting as a decoy activator of complement and by fostering B. burgdorferi replication at the transmission site.
Keywords: Borrelia burgdorferi; Ixodes scapularis; complement cascade; tick calreticulin.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


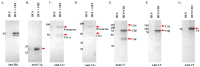

Similar articles
-
A tick saliva serpin, IxsS17 inhibits host innate immune system proteases and enhances host colonization by Lyme disease agent.PLoS Pathog. 2024 Feb 23;20(2):e1012032. doi: 10.1371/journal.ppat.1012032. eCollection 2024 Feb. PLoS Pathog. 2024. PMID: 38394332 Free PMC article.
-
Ixodes scapularis nymph saliva protein blocks host inflammation and complement-mediated killing of Lyme disease agent, Borrelia burgdorferi.Front Cell Infect Microbiol. 2023 Oct 26;13:1253670. doi: 10.3389/fcimb.2023.1253670. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37965264 Free PMC article.
-
Borrelia burgdorferi infection modifies protein content in saliva of Ixodes scapularis nymphs.BMC Genomics. 2021 Mar 4;22(1):152. doi: 10.1186/s12864-021-07429-0. BMC Genomics. 2021. PMID: 33663385 Free PMC article.
-
The role of Ixodes scapularis, Borrelia burgdorferi and wildlife hosts in Lyme disease prevalence: A quantitative review.Ticks Tick Borne Dis. 2018 Jul;9(5):1103-1114. doi: 10.1016/j.ttbdis.2018.04.006. Epub 2018 Apr 16. Ticks Tick Borne Dis. 2018. PMID: 29680260 Review.
-
Borrelia burgdorferi sensu lato prevalence in Ixodes scapularis from Canada: A thirty-year summary and meta-analysis (1990-2020).Acta Trop. 2024 Aug;256:107268. doi: 10.1016/j.actatropica.2024.107268. Epub 2024 May 22. Acta Trop. 2024. PMID: 38782109 Review.
References
-
- Sosa J.P., Ferreira Caceres M.M., Agadi K., Pandav K., Mehendale M., Mehta J.M., Go C.C., Matos W.F., Guntipalli P., Belizaire M.E. Diseases Transmitted by the Black-Legged Ticks in the United States: A Comprehensive Review of the Literature. Cureus. 2021;13:e17526. doi: 10.7759/cureus.17526. - DOI - PMC - PubMed
-
- Guzman N., Yarrarapu S.N.S., Beidas S.O. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2023. Anaplasma Phagocytophilum. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

