Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder
- PMID: 39056776
- PMCID: PMC11275104
- DOI: 10.3390/cells13141194
Human-Induced Pluripotent Stem Cell (iPSC)-Derived GABAergic Neuron Differentiation in Bipolar Disorder
Abstract
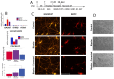
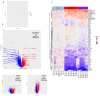
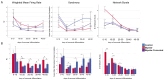
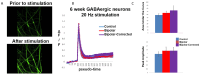
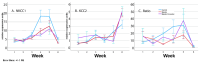
Bipolar disorder (BP) is a recurring psychiatric condition characterized by alternating episodes of low energy (depressions) followed by manias (high energy). Cortical network activity produced by GABAergic interneurons may be critical in maintaining the balance in excitatory/inhibitory activity in the brain during development. Initially, GABAergic signaling is excitatory; with maturation, these cells undergo a functional switch that converts GABAA channels from depolarizing (excitatory) to hyperpolarizing (inhibitory), which is controlled by the intracellular concentration of two chloride transporters. The earliest, NKCC1, promotes chloride entry into the cell and depolarization, while the second (KCC2) stimulates movement of chloride from the neuron, hyperpolarizing it. Perturbations in the timing or expression of NKCC1/KCC2 may affect essential morphogenetic events including cell proliferation, migration, synaptogenesis and plasticity, and thereby the structure and function of the cortex. We derived induced pluripotent stem cells (iPSC) from BP patients and undiagnosed control (C) individuals, then modified a differentiation protocol to form GABAergic interneurons, harvesting cells at sequential stages of differentiation. qRT-PCR and RNA sequencing indicated that after six weeks of differentiation, controls transiently expressed high levels of NKCC1. Using multi-electrode array (MEA) analysis, we observed that BP neurons exhibit increased firing, network bursting and decreased synchrony compared to C. Understanding GABA signaling in differentiation may identify novel approaches and new targets for treatment of neuropsychiatric disorders such as BP.
Keywords: GABA switch; KCC2; NKCC1; SLC12A2; SLC12A5; gamma-aminobutyric acid; neuron; patient; skin biopsy; somatostatin; stem cell.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Erratum: Eyestalk Ablation to Increase Ovarian Maturation in Mud Crabs.J Vis Exp. 2023 May 26;(195). doi: 10.3791/6561. J Vis Exp. 2023. PMID: 37235796
-
Histology, Axon.2022 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2022 Nov 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 32119275 Free Books & Documents.
-
GABAA-Receptor Signaling and Ionic Plasticity in the Generation and Spread of Seizures.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 6. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 6. PMID: 39637123 Free Books & Documents. Review.
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Hemophilia B.2000 Oct 2 [updated 2024 Jun 6]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2000 Oct 2 [updated 2024 Jun 6]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301668 Free Books & Documents. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

