Epacadostat plus pembrolizumab versus placebo plus pembrolizumab for advanced urothelial carcinoma: results from the randomized phase III ECHO-303/KEYNOTE-698 study
- PMID: 39054485
- PMCID: PMC11270759
- DOI: 10.1186/s12885-023-11213-6
Epacadostat plus pembrolizumab versus placebo plus pembrolizumab for advanced urothelial carcinoma: results from the randomized phase III ECHO-303/KEYNOTE-698 study
Abstract
Background: Indoleamine 2,3-dioxygenase 1 (IDO1) levels correlate with poor outcomes in urothelial carcinoma (UC). IDO1 and programmed death-ligand 1 (PD-L1) are often co-expressed. Epacadostat is a potent and highly selective inhibitor of IDO1. In a subgroup analysis of patients with advanced UC participating in a phase I/II study, epacadostat-pembrolizumab treatment produced an objective response rate (ORR) of 35%.
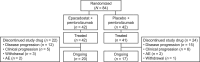
Methods: ECHO-303/KEYNOTE-698 was a double-blinded, randomized phase III study of adults with metastatic or unresectable locally advanced UC with recurrence or progression following first-line platinum-based chemotherapy. Participants were randomized to epacadostat 100 mg twice daily (BID) plus pembrolizumab or placebo plus pembrolizumab until completion of 35 pembrolizumab infusions, disease progression, or unacceptable toxicity. The primary endpoint was investigator-assessed ORR per Response Evaluation Criteria in Solid Tumors version 1.1.
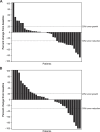
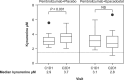
Results: Target enrollment was 648 patients; enrollment was halted early based on efficacy results from the phase III ECHO-301/KEYNOTE-252 study in metastatic melanoma. Forty-two patients were randomized to each treatment arm. Median duration of follow-up was 62 days in each arm. The investigator-assessed ORR (unconfirmed) was 26.2% (95% CI 16.35-48.11) for epacadostat plus pembrolizumab and 11.9% (95% CI 4.67-29.50) for placebo plus pembrolizumab. Two complete responses were reported, both in the placebo-plus-pembrolizumab arm. Circulating kynurenine levels increased from C1D1 to C2D1 in the placebo-plus-pembrolizumab arm and numerically decreased in the epacadostat-plus-pembrolizumab arm. The safety profile of epacadostat plus pembrolizumab was similar to that of pembrolizumab monotherapy, although a numerically greater proportion of patients in the combination vs. control arm experienced treatment-related grade ≥ 3 adverse events (16.7% vs. 7.3%). One patient in each arm died due to cardiovascular events, which were not deemed drug-related. No new safety concerns were identified for either agent.
Conclusions: Epacadostat plus pembrolizumab demonstrated anti-tumor activity and was generally tolerable as second-line treatment of patients with unresectable locally advanced or recurrent/progressive metastatic UC. Epacadostat 100 mg BID, when administered with pembrolizumab, did not normalize circulating kynurenine in most patients. Further study of combined IDO1/PD-L1 inhibition in this patient population, particularly with epacadostat doses that result in durable normalization of circulating kynurenine, may be warranted.
Trial registration: ClinicalTrials.gov, NCT03374488. Registered 12/15/2017.
Keywords: Epacadostat; IDO1; Immune checkpoint inhibition; PD-L1; Pembrolizumab; Randomized controlled study; Urothelial carcinoma.
© 2023. The Author(s).
Conflict of interest statement
IC is an advisory board member for AstraZeneca, Boehringer Ingelheim, Eli Lilly, F. Hoffmann-La Roche, MSD, and Pfizer; received personal fees for lectures from Eli Lilly, MSD, Novartis, Pfizer, Quintiles, and Roche; received funding to the institution to support trial conduct from Astellas, AstraZeneca, BMS, Boehringer Ingelheim, Eli Lilly, Merck Serono, MSD, Parexel, Pfizer, Quintiles, and Taiho. ERP reports grants from the Sponsor of trial described in this publication, during the conduct of the study; grants and personal fees from Genentech, AstraZeneca, Bristol-Myers Squibb, Merck, and Pfizer; grants from Peloton and Astellas; personal fees from Incyte, Seattle Genetics, Janssen, Flatiron Health, Infinity Pharma, and MEI Pharma; in addition, ERP has a patent, U.S. Patent Application No. 14/588,503, pending; and has received honoraria for CME certified presentations from the following entities: AUA, Clinical Care Options, Fox Chase Cancer Center, Georgetown, ASCO, Medscape, Mt Sinai Icahn School of Medicine, NCCN, Omniprex, OncLive, PER, PriME Oncology, Research to Practice, Spire Learning, University of Pennsylvania, Thomas Jefferson University, and the University of Michigan. HG received honoraria from Pfizer; served in a consulting or advisory role for Pfizer, Ipsen, Bristol-Myers Squibb, AstraZeneca, Janssen-Cilag, Merck Sharp & Dohme, and Roche; and has received support for travel/accommodations from AstraZeneca. RL has served in a consulting/advisory role for Pfizer, Bristol-Myers Squibb, MSD, Roche, Isotopia, AstraZeneca, Bayer, Astellas, and Janssen. BYA received personal fees for consulting, advisory boards, and lectures as well as personal grants and institutional grants for trials from: Astrazeneca, Astellas, Bayer, BMS, Eisai, Ferring, Janssen, Ipsen, Merck, MSD, Pfizer, Roche, and Sanofi. FXP is an advisory board member for Bayer and Janssen. AP has served in an advisory role for AstraZeneca, Bristol-Myers Squibb, Roche, MSD/Merck & Co., Janssen, Pfizer, Teva, Astellas, Bayer, and Eisai. AN reports honoraria from Roche, Merck, AstraZeneca, Janssen, BMS, and Foundation Medicine; a consulting or advisor role with Merck, Roche, Bayer, AstraZeneca, Clovis Oncology, Janssen, Incyte, Seattle Genetics/Astellas, Bristol-Myers Squibb, Rainier Therapeutics, Glaxo Smith Kline, and Ferring; research funding to the institution from Merck, Astra Zeneca, and Ipsen; support for travel/accommodation/expenses from Roche, Merck, AstraZeneca, Janssen, and Rainier Therapeutics; and spousal employment/stock ownership with Bayer. JB reports consultancy and lectures fee from: Merck, AstraZeneca, Genentech, Pfizer, and BMS. HN is an advisory board member for AstraZeneca, MSD, Ono, Bristol-Myers Squibb, Chugai, Taiho, and Janssen; received personal fees for lectures from MSD, Chugai, Ono, AstraZeneca, and Astellas; and received funding to the institution to support trial conduct from Ono and Chugai. JC and MM are salaried employees of and own stock in Incyte Corporation. RK and CJ are salaried employees of and own stock in Merck & Co., Inc., Rahway, NJ, USA. TP reports advisory and lecture fees from AstraZeneca, BMS, Exelixis, Incyte, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, Eisai, and Roche; grant/funding to Institution from: AstraZeneca, Roche, BMS, Exelixis, Ipsen, Merck, MSD, Novartis, Pfizer, Seattle Genetics, Merck Serono, Astellas, Johnson & Johnson, and Eisai; and travel/accommodation/expenses from Roche, Pfizer, MSD, AstraZeneca, and Ipsen. CNS has served as a consultant for Pfizer, Merck, MSD, AstraZeneca, Astellas Pharma, Sanofi-Genzyme, Roche/Genentech, Incyte, Medscape, Immunomedics, Clovis Oncology, and UroToday.
Figures
Similar articles
-
Pembrolizumab plus either epacadostat or placebo for cisplatin-ineligible urothelial carcinoma: results from the ECHO-307/KEYNOTE-672 study.BMC Cancer. 2024 Jul 25;23(Suppl 1):1252. doi: 10.1186/s12885-023-10727-3. BMC Cancer. 2024. PMID: 39054491 Free PMC article. Clinical Trial.
-
Pembrolizumab with platinum-based chemotherapy with or without epacadostat as first-line treatment for metastatic non-small cell lung cancer: a randomized, partially double-blind, placebo-controlled phase II study.BMC Cancer. 2024 Jul 25;23(Suppl 1):1250. doi: 10.1186/s12885-022-10427-4. BMC Cancer. 2024. PMID: 39054462 Free PMC article. Clinical Trial.
-
Epacadostat plus pembrolizumab versus placebo plus pembrolizumab as first-line treatment for metastatic non-small cell lung cancer with high levels of programmed death-ligand 1: a randomized, double-blind phase 2 study.BMC Cancer. 2024 Jul 25;23(Suppl 1):1251. doi: 10.1186/s12885-023-11203-8. BMC Cancer. 2024. PMID: 39054476 Free PMC article. Clinical Trial.
-
Pembrolizumab monotherapy versus chemotherapy for treatment of advanced urothelial carcinoma with disease progression during or following platinum-containing chemotherapy. A Cochrane Rapid Review.Cochrane Database Syst Rev. 2018 Jul 23;7(7):CD012838. doi: 10.1002/14651858.CD012838.pub2. Cochrane Database Syst Rev. 2018. PMID: 30036453 Free PMC article. Review.
-
[First-line treatment of metastatic urothelial carcinoma : Update immuno-oncology].Urologe A. 2020 Jul;59(7):797-803. doi: 10.1007/s00120-020-01235-4. Urologe A. 2020. PMID: 32500171 Review. German.
References
-
- Globocan. WHO International Agency for Research on Cancer Fact Sheets: Bladder. 2018. https://gco.iarc.fr/today/data/factsheets/cancers/30-Bladder-fact-sheet.pdf. Accessed 21 July 2020.
-
- Bellmunt J, Orsola A, Leow JJ, Wiegel T, De Santis M, Horwich A. Bladder cancer: ESMO practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii408.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Research Materials

