An Immunoreceptor-Targeting Strategy with Minimalistic C3b Peptide Fusion Enhances SARS-CoV-2 RBD mRNA Vaccine Immunogenicity
- PMID: 39050877
- PMCID: PMC11268571
- DOI: 10.2147/IJN.S463546
An Immunoreceptor-Targeting Strategy with Minimalistic C3b Peptide Fusion Enhances SARS-CoV-2 RBD mRNA Vaccine Immunogenicity
Abstract
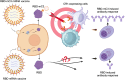
Introduction: The clinical success of mRNA vaccine during the COVID-19 pandemic has inspired emerging approaches to elevate mRNA vaccine immunogenicity. Among them, antigen fusion protein designs for improved immune cell targeting have been shown to augment humoral immunity against small antigen targets.
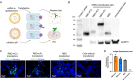
Methods: This research demonstrates that SARS-CoV-2 receptor binding domain (RBD) fusion with a minimalistic peptide segment of complement component 3b (C3b, residues 727-767) ligand can improve mRNA vaccine immunogenicity through antigen targeting to complement receptor 1 (CR1). We affirm vaccines' antigenicity and targeting ability towards specific receptors through Western blot and immunofluorescence assay. Furthermore, mice immunization studies help the investigation of the antibody responses.
Results: Using SARS-CoV-2 Omicron RBD antigen, we compare mRNA vaccine formulations expressing RBD fusion protein with mouse C3b peptide (RBD-mC3), RBD fusion protein with mouse Fc (RBD-Fc), and wild-type RBD. Our results confirm the proper antigenicity and normal functionality of RBD-mC3. Upon validating comparable antigen expression by the different vaccine formulations, receptor-targeting capability of the fusion antigens is further confirmed. In mouse immunization studies, we show that while both RBD-mC3 and RBD-Fc elevate vaccine immunogenicity, RBD-mC3 leads to more sustained RBD-specific titers over the RBD-Fc design, presumably due to reduced antigenic diversion by the minimalistic targeting ligand.
Conclusion: The study demonstrates a novel C3b-based antigen design strategy for immune cell targeting and mRNA vaccine enhancement.
Keywords: complement component 3b; complement receptor 1; follicular dendritic cells; immune cell targeting; nanotechnology; vaccinology.
© 2024 Chiu et al.
Conflict of interest statement
The authors declare no conflicts of interest in this research.
Figures

Similar articles
-
Development of nanoparticle vaccines utilizing designed Fc-binding homo-oligomers and RBD-Fc of SARS-CoV-2.Antiviral Res. 2024 Jul;227:105917. doi: 10.1016/j.antiviral.2024.105917. Epub 2024 May 21. Antiviral Res. 2024. PMID: 38782067
-
A chimeric mRNA vaccine of S-RBD with HA conferring broad protection against influenza and COVID-19 variants.PLoS Pathog. 2024 Sep 20;20(9):e1012508. doi: 10.1371/journal.ppat.1012508. eCollection 2024 Sep. PLoS Pathog. 2024. PMID: 39303003 Free PMC article.
-
High-Resolution Linear Epitope Mapping of the Receptor Binding Domain of SARS-CoV-2 Spike Protein in COVID-19 mRNA Vaccine Recipients.Microbiol Spectr. 2021 Dec 22;9(3):e0096521. doi: 10.1128/Spectrum.00965-21. Epub 2021 Nov 10. Microbiol Spectr. 2021. PMID: 34756082 Free PMC article.
-
Severe acute respiratory syndrome-coronavirus-2 spike (S) protein based vaccine candidates: State of the art and future prospects.Rev Med Virol. 2021 May;31(3):e2183. doi: 10.1002/rmv.2183. Epub 2020 Oct 15. Rev Med Virol. 2021. PMID: 33594794 Free PMC article. Review.
-
SARS-CoV-2-Specific Vaccine Candidates; the Contribution of Structural Vaccinology.Vaccines (Basel). 2022 Feb 3;10(2):236. doi: 10.3390/vaccines10020236. Vaccines (Basel). 2022. PMID: 35214693 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

