Bag-1-mediated HSF1 phosphorylation regulates expression of heat shock proteins in breast cancer cells
- PMID: 39049197
- PMCID: PMC11492399
- DOI: 10.1002/2211-5463.13843
Bag-1-mediated HSF1 phosphorylation regulates expression of heat shock proteins in breast cancer cells
Abstract
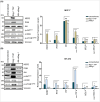
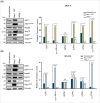
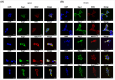
According to the World Health Organization in 2022, 2.3 million women were diagnosed with breast cancer. Investigating the interaction networks between Bcl-2-associated athanogene (Bag)-1 and other chaperone proteins may further the current understanding of the regulation of protein homeostasis in breast cancer cells and contribute to the development of treatment options. The present study aimed to determine the interactions between Bag-1 and heat shock proteins (HSPs); namely, HSP90, HSP70 and HSP27, to elucidate their role in promoting heat shock factor-1 (HSF1)-dependent survival of breast cancer cells. HER2-negative (MCF-7) and HER2-positive (BT-474) cell lines were used to examine the impact of Bag-1 expression on HSF1 and HSPs. We demonstrated that Bag-1 overexpression promoted HER2 expression in breast cancer cells, thereby resulting in the concurrent constitutive activation of the HSF1-HSP axis. The activation of HSP results in the stabilization of several tumor-promoting HSP clients such as AKT, mTOR and HSF1 itself, which substantially accelerates tumor development. Our results suggest that Bag-1 can modulate the chaperone activity of HSPs, such as HSP27, by directly or indirectly regulating the phosphorylation of HSF1. This modulation of chaperone activity can influence the activation of genes involved in cellular homeostasis, thereby protecting cells against stress.
Keywords: Bag‐1; HER2; HSF1; breast cancer; chaperones.
© 2024 The Author(s). FEBS Open Bio published by John Wiley & Sons Ltd on behalf of Federation of European Biochemical Societies.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures

Similar articles
-
HER2/ErbB2 activates HSF1 and thereby controls HSP90 clients including MIF in HER2-overexpressing breast cancer.Cell Death Dis. 2014 Jan 2;5(1):e980. doi: 10.1038/cddis.2013.508. Cell Death Dis. 2014. PMID: 24384723 Free PMC article.
-
Heat shock protein 25 or inducible heat shock protein 70 activates heat shock factor 1: dephosphorylation on serine 307 through inhibition of ERK1/2 phosphorylation.J Biol Chem. 2006 Jun 23;281(25):17220-17227. doi: 10.1074/jbc.M600062200. Epub 2006 Apr 19. J Biol Chem. 2006. PMID: 16624816
-
Sulphoraphane, a naturally occurring isothiocyanate induces apoptosis in breast cancer cells by targeting heat shock proteins.Biochem Biophys Res Commun. 2012 Oct 12;427(1):80-5. doi: 10.1016/j.bbrc.2012.09.006. Epub 2012 Sep 10. Biochem Biophys Res Commun. 2012. PMID: 22975350
-
Hsf1 on a leash - controlling the heat shock response by chaperone titration.Exp Cell Res. 2020 Nov 1;396(1):112246. doi: 10.1016/j.yexcr.2020.112246. Epub 2020 Aug 27. Exp Cell Res. 2020. PMID: 32861670 Review.
-
The Heat Shock Response as a Condensate Cascade.J Mol Biol. 2024 Jul 15;436(14):168642. doi: 10.1016/j.jmb.2024.168642. Epub 2024 Jun 5. J Mol Biol. 2024. PMID: 38848866 Review.
References
-
- Cutress RI, Townsend PA, Sharp A, Maison A, Wood L, Lee R, Brimmell M, Mullee MA, Johnson PW, Royle GT et al. (2003) The nuclear BAG‐1 isoform, BAG‐1L, enhances oestrogen‐dependent transcription. Oncogene 22, 4973–4982. - PubMed
-
- Schopf FH, Biebl MM and Buchner J (2017) The HSP90 chaperone machinery. Nat Rev Mol Cell Biol 18, 345–360. - PubMed
-
- Morán Luengo T, Mayer MP and Rüdiger SGD (2019) The HSP70‐HSP90 chaperone cascade in protein folding. Trends Cell Biol 29, 164–177. - PubMed
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

