Cytopathic effects in Mimivirus infection: understanding the kinetics of virus-cell interaction
- PMID: 39045993
- PMCID: PMC11285858
- DOI: 10.1590/0074-02760230186
Cytopathic effects in Mimivirus infection: understanding the kinetics of virus-cell interaction
Abstract
Background: Giant viruses have brought new insights into different aspects of virus-cell interactions. The resulting cytopathic effects from these interactions are one of the main aspects of infection assessment in a laboratory routine, mainly reflecting on the morphological features of an infected cell.
Objectives: In this work, we follow the entire kinetics of the cytopathic effect in cells infected by viruses of the Mimiviridae family, spatiotemporally quantifying typical features such as cell roundness, loss of motility, decrease in cell area and cell lysis.
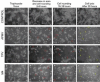
Methods: Infections by Acanthamoeba polyphaga mimivirus (APMV), Tupanvirus (TPV) and M4 were carried out at multiplicity of infection (MOI) 1 and MOI 10 in Acanthamoeba castellanii. Monitoring of infections was carried out using time lapse microscopy for up to 72 hours. The images were analyzed using ImageJ software.
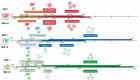
Findings: The data obtained indicate that APMV is the slowest virus in inducing the cytopathic effects of rounding, decrease in cell area, mobility and cell lysis. However, it is the only virus whose MOI increase accelerates the lysis process of infected cells. In turn, TPV and M4 rapidly induce morphological and behavioral changes.
Main conclusions: Our results indicate that mimiviruses induce different temporal responses within the host cell and that it is possible to use these kinetic data to facilitate the understanding of infection by these viruses.
Conflict of interest statement
All authors have read and agreed to the published version of the manuscript
Figures
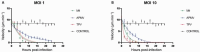
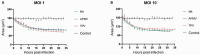


Similar articles
-
Kinetic Analysis of Acanthamoeba castellanii Infected with Giant Viruses Quantitatively Revealed Process of Morphological and Behavioral Changes in Host Cells.Microbiol Spectr. 2021 Sep 3;9(1):e0036821. doi: 10.1128/Spectrum.00368-21. Epub 2021 Aug 25. Microbiol Spectr. 2021. PMID: 34431709 Free PMC article.
-
Amoebal Tubulin Cleavage Late during Infection Is a Characteristic Feature of Mimivirus but Not of Marseillevirus.Microbiol Spectr. 2022 Dec 21;10(6):e0275322. doi: 10.1128/spectrum.02753-22. Epub 2022 Dec 1. Microbiol Spectr. 2022. PMID: 36453900 Free PMC article.
-
Mimivirus Fibrils Are Important for Viral Attachment to the Microbial World by a Diverse Glycoside Interaction Repertoire.J Virol. 2015 Dec;89(23):11812-9. doi: 10.1128/JVI.01976-15. Epub 2015 Sep 16. J Virol. 2015. PMID: 26378162 Free PMC article.
-
Acanthamoeba polyphaga mimivirus and other giant viruses: an open field to outstanding discoveries.Virol J. 2014 Jun 30;11:120. doi: 10.1186/1743-422X-11-120. Virol J. 2014. PMID: 24976356 Free PMC article. Review.
-
Mimivirus: leading the way in the discovery of giant viruses of amoebae.Nat Rev Microbiol. 2017 Apr;15(4):243-254. doi: 10.1038/nrmicro.2016.197. Epub 2017 Feb 27. Nat Rev Microbiol. 2017. PMID: 28239153 Free PMC article. Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources

