AAV-NRIP gene therapy ameliorates motor neuron degeneration and muscle atrophy in ALS model mice
- PMID: 39044305
- PMCID: PMC11267858
- DOI: 10.1186/s13395-024-00349-z
AAV-NRIP gene therapy ameliorates motor neuron degeneration and muscle atrophy in ALS model mice
Abstract
Background: Amyotrophic lateral sclerosis (ALS) is characterized by progressive motor neuron (MN) degeneration, leading to neuromuscular junction (NMJ) dismantling and severe muscle atrophy. The nuclear receptor interaction protein (NRIP) functions as a multifunctional protein. It directly interacts with calmodulin or α-actinin 2, serving as a calcium sensor for muscle contraction and maintaining sarcomere integrity. Additionally, NRIP binds with the acetylcholine receptor (AChR) for NMJ stabilization. Loss of NRIP in muscles results in progressive motor neuron degeneration with abnormal NMJ architecture, resembling ALS phenotypes. Therefore, we hypothesize that NRIP could be a therapeutic factor for ALS.
Methods: We used SOD1 G93A mice, expressing human SOD1 with the ALS-linked G93A mutation, as an ALS model. An adeno-associated virus vector encoding the human NRIP gene (AAV-NRIP) was generated and injected into the muscles of SOD1 G93A mice at 60 days of age, before disease onset. Pathological and behavioral changes were measured to evaluate the therapeutic effects of AAV-NRIP on the disease progression of SOD1 G93A mice.
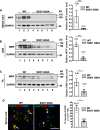
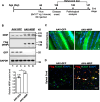
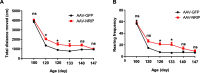
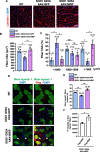
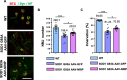
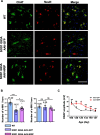
Results: SOD1 G93A mice exhibited lower NRIP expression than wild-type mice in both the spinal cord and skeletal muscle tissues. Forced NRIP expression through AAV-NRIP intramuscular injection was observed in skeletal muscles and retrogradely transduced into the spinal cord. AAV-NRIP gene therapy enhanced movement distance and rearing frequencies in SOD1 G93A mice. Moreover, AAV-NRIP increased myofiber size and slow myosin expression, ameliorated NMJ degeneration and axon terminal denervation at NMJ, and increased the number of α-motor neurons (α-MNs) and compound muscle action potential (CMAP) in SOD1 G93A mice.
Conclusions: AAV-NRIP gene therapy ameliorates muscle atrophy, motor neuron degeneration, and axon terminal denervation at NMJ, leading to increased NMJ transmission and improved motor functions in SOD1 G93A mice. Collectively, AAV-NRIP could be a potential therapeutic drug for ALS.
Keywords: AAV; ALS; Gene therapy; Motor neuron; Muscle; NRIP; Neuromuscular junction; SOD1 G93A.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Electroacupuncture alleviates motor dysfunction by regulating neuromuscular junction disruption and neuronal degeneration in SOD1G93A mice.Brain Res Bull. 2024 Oct 1;216:111036. doi: 10.1016/j.brainresbull.2024.111036. Epub 2024 Jul 29. Brain Res Bull. 2024. PMID: 39084570
-
Muscle-restricted nuclear receptor interaction protein knockout causes motor neuron degeneration through down-regulation of myogenin at the neuromuscular junction.J Cachexia Sarcopenia Muscle. 2018 Aug;9(4):771-785. doi: 10.1002/jcsm.12299. Epub 2018 Apr 2. J Cachexia Sarcopenia Muscle. 2018. PMID: 29608040 Free PMC article.
-
Axonal degeneration, distal collateral branching and neuromuscular junction architecture alterations occur prior to symptom onset in the SOD1(G93A) mouse model of amyotrophic lateral sclerosis.J Chem Neuroanat. 2016 Oct;76(Pt A):35-47. doi: 10.1016/j.jchemneu.2016.03.003. Epub 2016 Mar 30. J Chem Neuroanat. 2016. PMID: 27038603
-
Therapeutics Targeting Skeletal Muscle in Amyotrophic Lateral Sclerosis.Biomolecules. 2024 Jul 22;14(7):878. doi: 10.3390/biom14070878. Biomolecules. 2024. PMID: 39062592 Free PMC article. Review.
-
Current potential pathogenic mechanisms of copper-zinc superoxide dismutase 1 (SOD1) in amyotrophic lateral sclerosis.Rev Neurosci. 2024 Feb 22;35(5):549-563. doi: 10.1515/revneuro-2024-0010. Print 2024 Jul 26. Rev Neurosci. 2024. PMID: 38381656 Review.
Cited by
-
Designing and optimizing AAV-mediated gene therapy for neurodegenerative diseases: from bench to bedside.J Transl Med. 2024 Sep 27;22(1):866. doi: 10.1186/s12967-024-05661-2. J Transl Med. 2024. PMID: 39334366 Free PMC article. Review.
-
Advancements in Drug Delivery Systems for the Treatment of Sarcopenia: An Updated Overview.Int J Mol Sci. 2024 Oct 7;25(19):10766. doi: 10.3390/ijms251910766. Int J Mol Sci. 2024. PMID: 39409095 Free PMC article. Review.
References
-
- Rothstein JD. Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol. 2009;65 Suppl 1:S3–9. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

