Thermosensitive Polyhedral Oligomeric Silsesquioxane Hybrid Hydrogel Enhances the Antibacterial Efficiency of Erythromycin in Bacterial Keratitis
- PMID: 39040621
- PMCID: PMC11260774
- DOI: 10.34133/bmr.0033
Thermosensitive Polyhedral Oligomeric Silsesquioxane Hybrid Hydrogel Enhances the Antibacterial Efficiency of Erythromycin in Bacterial Keratitis
Abstract
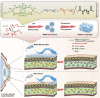
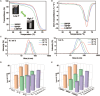
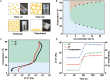
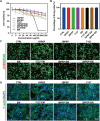
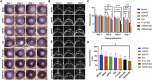
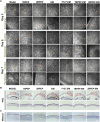
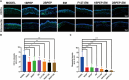
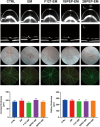
Bacterial keratitis is a serious ocular infection that can impair vision or even cause blindness. The clinical use of antibiotics is limited due to their low bioavailability and drug resistance. Hence, there is a need to develop a novel drug delivery system for this infectious disease. In this study, erythromycin (EM) was encapsulated into a bifunctional polyhedral oligomeric silsesquioxane (BPOSS) with the backbone of the poly-PEG/PPG urethane (BPEP) hydrogel with the aim of improving the drug efficiency in treating bacterial keratitis. A comprehensive characterization of the BPEP hydrogel was performed, and its biocompatibility was assessed. Furthermore, we carried out the evaluation of the antimicrobial effect of the BPEP-EM hydrogel in S. aureus keratitis using in vivo mouse model. The BPEP hydrogel exhibited self-assembling and thermogelling properties, which assisted the drug loading of drug EM and improved its water solubility. Furthermore, the BPEP hydrogel could effectively bind with mucin on the ocular surface, thereby markedly prolonging the ocular residence time of EM. In vivo testing confirmed that the BPEP-EM hydrogel exerted a potent therapeutic action in the mouse model of bacterial keratitis. In addition, the hydrogel also exhibited an excellent biocompatibility. Our findings demonstrate that the BPEP-EM hydrogel showed a superior therapeutic effect in bacterial keratitis and demonstrated its potential as an ophthalmic formulation.
Copyright © 2024 Lan Zheng et al.
Conflict of interest statement
Competing interests: The authors declare that they have no competing interests.
Figures


Similar articles
-
Effectiveness of an ocular adhesive polyhedral oligomeric silsesquioxane hybrid thermo-responsive FK506 hydrogel in a murine model of dry eye.Bioact Mater. 2021 Jul 28;9:77-91. doi: 10.1016/j.bioactmat.2021.07.027. eCollection 2022 Mar. Bioact Mater. 2021. PMID: 34820557 Free PMC article.
-
A Hydrogel-Based Hybrid Theranostic Contact Lens for Fungal Keratitis.ACS Nano. 2016 Jul 26;10(7):6464-73. doi: 10.1021/acsnano.6b00601. Epub 2016 Jun 3. ACS Nano. 2016. PMID: 27244244
-
Ferrous sulfate-loaded hydrogel cures Staphylococcus aureus infection via facilitating a ferroptosis-like bacterial cell death in a mouse keratitis model.Biomaterials. 2022 Nov;290:121842. doi: 10.1016/j.biomaterials.2022.121842. Epub 2022 Sep 30. Biomaterials. 2022. PMID: 36206665
-
Review: Emerging strategies for antimicrobial drug delivery to the ocular surface: Implications for infectious keratitis.Ocul Surf. 2017 Oct;15(4):670-679. doi: 10.1016/j.jtos.2017.06.001. Epub 2017 Jun 7. Ocul Surf. 2017. PMID: 28602948 Review.
-
Pharmacokinetic considerations in the treatment of bacterial keratitis.Clin Pharmacokinet. 1994 Aug;27(2):129-49. doi: 10.2165/00003088-199427020-00005. Clin Pharmacokinet. 1994. PMID: 7955776 Review.
References
-
- Tuft S, Somerville TF, Li JO, Neal T, De S, Horsburgh MJ, Fothergill JL, Foulkes D, Kaye S. Bacterial keratitis: Identifying the areas of clinical uncertainty. Prog Retin Eye Res. 2022;89:101031. - PubMed
-
- Miller D, Cavuoto KM, Alfonso EC. Bacterial keratitis, in Infections of the cornea and conjunctiva. Singapore: Springer Singapore; 2021, p. 85–104.
LinkOut - more resources
Full Text Sources

