Compartmentalization of the inflammatory response during bacterial sepsis and severe COVID-19
- PMID: 39035623
- PMCID: PMC11258514
- DOI: 10.1016/j.jointm.2024.01.001
Compartmentalization of the inflammatory response during bacterial sepsis and severe COVID-19
Abstract
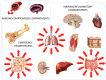
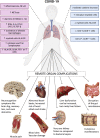
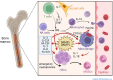
Acute infections cause local and systemic disorders which can lead in the most severe forms to multi-organ failure and eventually to death. The host response to infection encompasses a large spectrum of reactions with a concomitant activation of the so-called inflammatory response aimed at fighting the infectious agent and removing damaged tissues or cells, and the anti-inflammatory response aimed at controlling inflammation and initiating the healing process. Fine-tuning at the local and systemic levels is key to preventing local and remote injury due to immune system activation. Thus, during bacterial sepsis and Coronavirus disease 2019 (COVID-19), concomitant systemic and compartmentalized pro-inflammatory and compensatory anti-inflammatory responses are occurring. Immune cells (e.g., macrophages, neutrophils, natural killer cells, and T-lymphocytes), as well as endothelial cells, differ from one compartment to another and contribute to specific organ responses to sterile and microbial insult. Furthermore, tissue-specific microbiota influences the local and systemic response. A better understanding of the tissue-specific immune status, the organ immunity crosstalk, and the role of specific mediators during sepsis and COVID-19 can foster the development of more accurate biomarkers for better diagnosis and prognosis and help to define appropriate host-targeted treatments and vaccines in the context of precision medicine.
Keywords: Acute respiratory distress syndrome; Bone marrow; Cytokines; Lungs; Mucosa; Vaccines.
© 2024 The Author(s).
Figures
Similar articles
-
Compartmentalization of the inflammatory response in sepsis and SIRS.J Endotoxin Res. 2006;12(3):151-70. doi: 10.1179/096805106X102246. J Endotoxin Res. 2006. PMID: 16719987 Review.
-
During Sepsis and COVID-19, the Pro-Inflammatory and Anti-Inflammatory Responses Are Concomitant.Clin Rev Allergy Immunol. 2023 Oct;65(2):183-187. doi: 10.1007/s12016-023-08965-1. Epub 2023 Jul 3. Clin Rev Allergy Immunol. 2023. PMID: 37395985 Review.
-
Coronavirus-19 (SARS-CoV-2) induces acute severe lung inflammation via IL-1 causing cytokine storm in COVID-19: a promising inhibitory strategy.J Biol Regul Homeost Agents. 2020 Nov-Dec;34(6):1971-1975. doi: 10.23812/20-1-E. J Biol Regul Homeost Agents. 2020. PMID: 33016027
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Bayesian network analysis of multi-compartmentalized immune responses in a murine model of sepsis and direct lung injury.BMC Res Notes. 2015 Sep 30;8:516. doi: 10.1186/s13104-015-1488-y. BMC Res Notes. 2015. PMID: 26423575 Free PMC article.
Cited by
-
Rapid and Robust Identification of Sepsis Using SeptiCyte RAPID in a Heterogeneous Patient Population.J Clin Med. 2024 Oct 10;13(20):6044. doi: 10.3390/jcm13206044. J Clin Med. 2024. PMID: 39457994 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources

