Ca2+ transients on the T cell surface trigger rapid integrin activation in a timescale of seconds
- PMID: 39033133
- PMCID: PMC11271479
- DOI: 10.1038/s41467-024-50464-0
Ca2+ transients on the T cell surface trigger rapid integrin activation in a timescale of seconds
Abstract
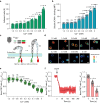
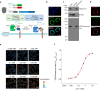
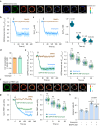
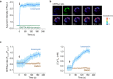
One question in lymphocyte homing is how integrins are rapidly activated to enable immediate arrest of fast rolling lymphocytes upon encountering chemokines at target vascular beds given the slow chemokine-induced integrin inside-out activation. Herein we demonstrate that chemokine CCL25-triggered Ca2+ influx induces T cell membrane-proximal external Ca2+ concentration ([Ca2+]ex) drop in 6 s from physiological concentration 1.2 mM to 0.3 mM, a critical extracellular Ca2+ threshold for inducing αLβ2 activation, triggering rapid αLβ2 activation and T cell arrest before occurrence of αLβ2 inside-out activation. Talin knockdown inhibits the slow inside-out activation of αLβ2 but not [Ca2+]ex drop-triggered αLβ2 quick activation. Blocking Ca2+ influx significantly suppresses T cell rolling-to-arrest transition and homing to skin lesions in a mouse psoriasis model, thus alleviating skin inflammation. [Ca2+]ex decrease-triggered rapid integrin activation bridges the gap between initial chemokine stimulation and slow integrin inside-out activation, ensuring immediate lymphocyte arrest and subsequent diapedesis on the right location.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
CBAP functions as a novel component in chemokine-induced ZAP70-mediated T-cell adhesion and migration.PLoS One. 2013 Apr 19;8(4):e61761. doi: 10.1371/journal.pone.0061761. Print 2013. PLoS One. 2013. PMID: 23620790 Free PMC article.
-
Th1 Cells Rolling on Selectins Trigger DAP12-Dependent Signals That Activate Integrin αLβ2.J Immunol. 2020 Jan 1;204(1):37-48. doi: 10.4049/jimmunol.1900680. Epub 2019 Nov 22. J Immunol. 2020. PMID: 31757864 Free PMC article.
-
Structural Basis of β2 Integrin Inside-Out Activation.Cells. 2022 Sep 28;11(19):3039. doi: 10.3390/cells11193039. Cells. 2022. PMID: 36231001 Free PMC article. Review.
-
The cytosolic protein talin induces an intermediate affinity integrin alphaLbeta2.J Biol Chem. 2007 Aug 17;282(33):24310-9. doi: 10.1074/jbc.M701860200. Epub 2007 Jun 25. J Biol Chem. 2007. PMID: 17591777
-
Right on the spot. Chemokine triggering of integrin-mediated arrest of rolling leukocytes.Thromb Haemost. 2006 Jan;95(1):5-11. Thromb Haemost. 2006. PMID: 16543955 Review.
References
MeSH terms
Substances
Grants and funding
- 31830112/National Natural Science Foundation of China (National Science Foundation of China)
- 32030024/National Natural Science Foundation of China (National Science Foundation of China)
- 82171804/National Natural Science Foundation of China (National Science Foundation of China)
- 92369102/National Natural Science Foundation of China (National Science Foundation of China)
- 32170769/National Natural Science Foundation of China (National Science Foundation of China)
LinkOut - more resources
Full Text Sources
Miscellaneous

