The impact of non- and anthracycline-based chemotherapy on fatigue in breast cancer survivors: results from WF-97415
- PMID: 39028321
- PMCID: PMC11271320
- DOI: 10.1007/s00520-024-08717-7
The impact of non- and anthracycline-based chemotherapy on fatigue in breast cancer survivors: results from WF-97415
Abstract
Purpose: To examine the differential effect of non- and anthracycline-based chemotherapy on fatigue over 12 months post-diagnosis among breast cancer survivors.
Methods: This study is based on a prospective Wake Forest NCI Community Oncology Research Program (NCORP) multicenter cohort study (WF-97415) of women with stage I to III breast cancer and non-cancer controls. Analyses compared those: 1) receiving, or 2) not receiving anthracycline chemotherapy, 3) receiving aromatase inhibitors (AIs) without chemotherapy, with 4) a comparator group without a history of cancer. In-person clinic assessments were conducted at: baseline (prior to chemotherapy or start of AI therapy), and 3 and 12 months after baseline. The Functional Assessment of Chronic Illness Therapy-Fatigue scale was the primary outcome. Estimated least squares means by group using mixed models with a random subject effect, fixed effects of time and group, and the interaction between time and group was used to compare groups across time, controlling for age, comorbidities, and treatment variables.
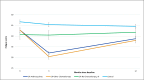
Results: Among 284 women (mean age = 53.4 years, sd 11.9 years), there was a significant (p < 0.0001) group by time interaction, with a sharp increase in fatigue at 3 months in the two chemotherapy groups in comparison to the non-chemotherapy and non-cancer controls. The two chemotherapy groups did not significantly differ in fatigue at any time point.
Conclusion: Women with breast cancer who receive non- or anthracycline-based chemotherapy experience similar trends in and levels of fatigue within the first year of treatment and greater fatigue than women receiving AIs alone or women without breast cancer.
Keywords: Anthracycline; Breast neoplasm; Chemotherapy; Fatigue; Longitudinal.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Prolonged impact of chemotherapy on fatigue in breast cancer survivors: a longitudinal comparison with radiotherapy-treated breast cancer survivors and noncancer controls.Cancer. 2012 Aug 1;118(15):3833-41. doi: 10.1002/cncr.26226. Epub 2011 Nov 15. Cancer. 2012. PMID: 22086766 Free PMC article.
-
Prevalence and risk factors for fatigue among breast cancer survivors on aromatase inhibitors.Eur J Cancer. 2018 Sep;101:47-54. doi: 10.1016/j.ejca.2018.06.009. Epub 2018 Jul 14. Eur J Cancer. 2018. PMID: 30014974 Free PMC article.
-
Effect of standard low-dose anthracycline chemotherapy on late congestive heart failure in breast cancer survivors aged between 50 and 59 at diagnosis: A nationwide study.Breast. 2020 Oct;53:125-129. doi: 10.1016/j.breast.2020.07.006. Epub 2020 Jul 31. Breast. 2020. PMID: 32771950 Free PMC article.
-
Anthracycline Use for Early Stage Breast Cancer in the Modern Era: a Review.Curr Treat Options Oncol. 2018 May 11;19(6):30. doi: 10.1007/s11864-018-0547-8. Curr Treat Options Oncol. 2018. PMID: 29752560 Review.
-
Trastuzumab-containing regimens for metastatic breast cancer.Cochrane Database Syst Rev. 2014 Jun 12;2014(6):CD006242. doi: 10.1002/14651858.CD006242.pub2. Cochrane Database Syst Rev. 2014. PMID: 24919460 Free PMC article. Review.
References
-
- Bower JE, Bak K, Berger A, Breitbart W, Escalante CP, Ganz PA et al (2014) Screening, assessment, and management of fatigue in adult survivors of cancer: an American Society of Clinical oncology clinical practice guideline adaptation. J Clin Oncol 32(17):1840–1850. 10.1200/jco.2013.53.4495 10.1200/jco.2013.53.4495 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical