Engineered exosomes as a prospective therapy for diabetic foot ulcers
- PMID: 39026930
- PMCID: PMC11255484
- DOI: 10.1093/burnst/tkae023
Engineered exosomes as a prospective therapy for diabetic foot ulcers
Abstract
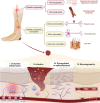
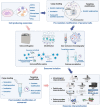
Diabetic foot ulcer (DFU), characterized by high recurrence rate, amputations and mortality, poses a significant challenge in diabetes management. The complex pathology involves dysregulated glucose homeostasis leading to systemic and local microenvironmental complications, including peripheral neuropathy, micro- and macro-angiopathy, recurrent infection, persistent inflammation and dysregulated re-epithelialization. Novel approaches to accelerate DFU healing are actively pursued, with a focus on utilizing exosomes. Exosomes are natural nanovesicles mediating cellular communication and containing diverse functional molecular cargos, including DNA, mRNA, microRNA (miRNA), lncRNA, proteins, lipids and metabolites. While some exosomes show promise in modulating cellular function and promoting ulcer healing, their efficacy is limited by low yield, impurities, low loading content and inadequate targeting. Engineering exosomes to enhance their curative activity represents a potentially more efficient approach for DFUs. This could facilitate focused repair and regeneration of nerves, blood vessels and soft tissue after ulcer development. This review provides an overview of DFU pathogenesis, strategies for exosome engineering and the targeted therapeutic application of engineered exosomes in addressing critical pathological changes associated with DFUs.
Keywords: Diabetic angiopathy; Diabetic foot; Diabetic peripheral neuropathy; Engineered exosomes; Foot ulcer; Inflammation; Re-epithelialization; Wound healing; Wound infection.
© The Author(s) 2024. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures

Similar articles
-
Mesenchymal Stromal Cell-Derived Tailored Exosomes Treat Bacteria-Associated Diabetes Foot Ulcers: A Customized Approach From Bench to Bed.Front Microbiol. 2021 Jul 27;12:712588. doi: 10.3389/fmicb.2021.712588. eCollection 2021. Front Microbiol. 2021. PMID: 34385994 Free PMC article. Review.
-
The MSC-Derived Exosomal lncRNA H19 Promotes Wound Healing in Diabetic Foot Ulcers by Upregulating PTEN via MicroRNA-152-3p.Mol Ther Nucleic Acids. 2020 Mar 6;19:814-826. doi: 10.1016/j.omtn.2019.11.034. Epub 2019 Dec 14. Mol Ther Nucleic Acids. 2020. PMID: 31958697 Free PMC article.
-
Exosomal ncRNAs: The pivotal players in diabetic wound healing.Front Immunol. 2022 Nov 7;13:1005307. doi: 10.3389/fimmu.2022.1005307. eCollection 2022. Front Immunol. 2022. PMID: 36420273 Free PMC article. Review.
-
Current scenario of traditional medicines in management of diabetic foot ulcers: A review.World J Diabetes. 2023 Jan 15;14(1):1-16. doi: 10.4239/wjd.v14.i1.1. World J Diabetes. 2023. PMID: 36684382 Free PMC article. Review.
-
Exosomes from adipose-derived stem cells overexpressing Nrf2 accelerate cutaneous wound healing by promoting vascularization in a diabetic foot ulcer rat model.Exp Mol Med. 2018 Apr 13;50(4):1-14. doi: 10.1038/s12276-018-0058-5. Exp Mol Med. 2018. PMID: 29651102 Free PMC article.
Cited by
-
Analysis of clinical characteristics in patients with diabetic foot ulcers undergoing amputation and establishment of a nomogram prediction model.Sci Rep. 2024 Nov 14;14(1):27934. doi: 10.1038/s41598-024-78215-7. Sci Rep. 2024. PMID: 39537768 Free PMC article.
References
-
- Walsh JW, Hoffstad OJ, Sullivan MO, Margolis DJ. Association of diabetic foot ulcer and death in a population-based cohort from the United Kingdom. Diabet Med. 2016;33:1493–8. - PubMed
-
- Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31:817–36. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

