The DNA double-strand break repair proteins γH2AX, RAD51, BRCA1, RPA70, KU80, and XRCC4 exhibit follicle-specific expression differences in the postnatal mouse ovaries from early to older ages
- PMID: 39023827
- PMCID: PMC11405603
- DOI: 10.1007/s10815-024-03189-4
The DNA double-strand break repair proteins γH2AX, RAD51, BRCA1, RPA70, KU80, and XRCC4 exhibit follicle-specific expression differences in the postnatal mouse ovaries from early to older ages
Abstract
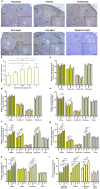
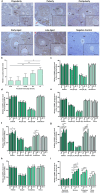
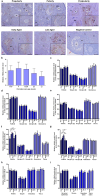
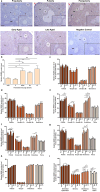
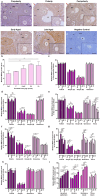
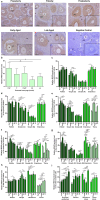
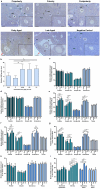
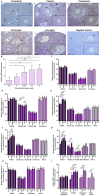
Purpose: Ovarian aging is closely related to a decrease in follicular reserve and oocyte quality. The precise molecular mechanisms underlying these reductions have yet to be fully elucidated. Herein, we examine spatiotemporal distribution of key proteins responsible for DNA double-strand break (DSB) repair in ovaries from early to older ages. Functional studies have shown that the γH2AX, RAD51, BRCA1, and RPA70 proteins play indispensable roles in HR-based repair pathway, while the KU80 and XRCC4 proteins are essential for successfully operating cNHEJ pathway.
Methods: Female Balb/C mice were divided into five groups as follows: Prepuberty (3 weeks old; n = 6), puberty (7 weeks old; n = 7), postpuberty (18 weeks old; n = 7), early aged (52 weeks old; n = 7), and late aged (60 weeks old; n = 7). The expression of DSB repair proteins, cellular senescence (β-GAL) and apoptosis (cCASP3) markers was evaluated in the ovaries using immunohistochemistry.
Result: β-GAL and cCASP3 levels progressively increased from prepuberty to aged groups (P < 0.05). Notably, γH2AX levels varied in preantral and antral follicles among the groups (P < 0.05). In aged groups, RAD51, BRCA1, KU80, and XRCC4 levels increased (P < 0.05), while RPA70 levels decreased (P < 0.05) compared to the other groups.
Conclusions: The observed alterations were primarily attributed to altered expression in oocytes and granulosa cells of the follicles and other ovarian cells. As a result, the findings indicate that these DSB repair proteins may play a role in the repair processes and even other related cellular events in ovarian cells from early to older ages.
Keywords: DNA double-strand break; Female infertility; Follicles; HR repair; Oocytes; Ovarian aging; cNHEJ.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

