NEO212, temozolomide conjugated to NEO100, exerts superior therapeutic activity over temozolomide in preclinical chemoradiation models of glioblastoma
- PMID: 39022643
- PMCID: PMC11252566
- DOI: 10.1093/noajnl/vdae095
NEO212, temozolomide conjugated to NEO100, exerts superior therapeutic activity over temozolomide in preclinical chemoradiation models of glioblastoma
Abstract
Background: The chemotherapeutic standard of care for patients with glioblastoma (GB) is radiation therapy (RT) combined with temozolomide (TMZ). However, during the twenty years since its introduction, this so-called Stupp protocol has revealed major drawbacks, because nearly half of all GBs harbor intrinsic treatment resistance mechanisms. Prime among these are the increased expression of the DNA repair protein O6-guanine-DNA methyltransferase (MGMT) and cellular deficiency in DNA mismatch repair (MMR). Patients with such tumors receive very little, if any, benefit from TMZ. We are developing a novel molecule, NEO212 (TMZ conjugated to NEO100), that harbors the potential to overcome these limitations.
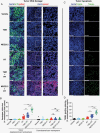
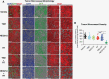
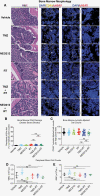
Methods: We used mouse models that were orthotopically implanted with GB cell lines or primary, radioresistant human GB stem cells, representing different treatment resistance mechanisms. Animals received NEO212 (or TMZ for comparison) without or with RT. Overall survival was recorded, and histology studies quantified DNA damage, apoptosis, microvessel density, and impact on bone marrow.
Results: In all tumor models, replacing TMZ with NEO212 in a schedule designed to mimic the Stupp protocol achieved a strikingly superior extension of survival, especially in TMZ-resistant and RT-resistant models. While NEO212 displayed pronounced radiation-sensitizing, DNA-damaging, pro-apoptotic, and anti-angiogenic effects in tumor tissue, it did not cause bone marrow toxicity.
Conclusions: NEO212 is a candidate drug to potentially replace TMZ within the standard Stupp protocol. It has the potential to become the first chemotherapeutic agent to significantly extend overall survival in TMZ-resistant patients when combined with radiation.
Keywords: O6-guanine-DNA methyltransferase; chemoradiation; mismatch repair deficiency; radiosensitization; temozolomide.
© The Author(s) 2024. Published by Oxford University Press, the Society for Neuro-Oncology and the European Association of Neuro-Oncology.
Conflict of interest statement
T.C.C. is founder and stakeholder of NeOnc Technologies, Los Angeles, California, USA. All other authors declare no conflict of interest.
Figures


Similar articles
-
Developing a clinically relevant radiosensitizer for temozolomide-resistant gliomas.PLoS One. 2020 Sep 3;15(9):e0238238. doi: 10.1371/journal.pone.0238238. eCollection 2020. PLoS One. 2020. PMID: 32881880 Free PMC article.
-
NEO212, a Perillyl Alcohol-Temozolomide Conjugate, Triggers Macrophage Differentiation of Acute Myeloid Leukemia Cells and Blocks Their Tumorigenicity.Cancers (Basel). 2022 Dec 9;14(24):6065. doi: 10.3390/cancers14246065. Cancers (Basel). 2022. PMID: 36551551 Free PMC article.
-
A novel temozolomide analog, NEO212, with enhanced activity against MGMT-positive melanoma in vitro and in vivo.Cancer Lett. 2015 Mar 28;358(2):144-151. doi: 10.1016/j.canlet.2014.12.021. Epub 2014 Dec 15. Cancer Lett. 2015. PMID: 25524552
-
Temozolomide resistance in glioblastoma multiforme.Genes Dis. 2016 May 11;3(3):198-210. doi: 10.1016/j.gendis.2016.04.007. eCollection 2016 Sep. Genes Dis. 2016. PMID: 30258889 Free PMC article. Review.
-
In search of effective therapies to overcome resistance to Temozolomide in brain tumours.Cancer Drug Resist. 2019 Dec 19;2(4):1018-1031. doi: 10.20517/cdr.2019.64. eCollection 2019. Cancer Drug Resist. 2019. PMID: 35582280 Free PMC article. Review.
References
-
- Stupp R, Mason WP, van den Bent MJ, et al.; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. - PubMed
-
- McMahon DJ, Gleeson JP, O’Reilly S, Bambury RM.. Management of newly diagnosed glioblastoma multiforme: Current state of the art and emerging therapeutic approaches. Med Oncol. 2022;39(9):129. - PubMed
-
- Hegi ME, Diserens AC, Gorlia T, et al.. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
