Stochasticity of anticancer mechanisms underlying clinical effectiveness of vorinostat
- PMID: 39021957
- PMCID: PMC11253278
- DOI: 10.1016/j.heliyon.2024.e33052
Stochasticity of anticancer mechanisms underlying clinical effectiveness of vorinostat
Abstract
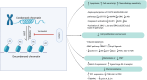
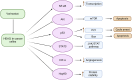
The Food and Drug Administration (FDA) has approved vorinostat, also called Zolinza®, for its effectiveness in fighting cancer. This drug is a suberoyl-anilide hydroxamic acid belonging to the class of histone deacetylase inhibitors (HDACis). Its HDAC inhibitory potential allows it to accumulate acetylated histones. This, in turn, can restore normal gene expression in cancer cells and activate multiple signaling pathways. Experiments have proven that vorinostat induces histone acetylation and cytotoxicity in many cancer cell lines, increases the level of p21 cell cycle proteins, and enhances pro-apoptotic factors while decreasing anti-apoptotic factors. Additionally, it regulates the immune response by up-regulating programmed death-ligand 1 (PD-L1) and interferon gamma receptor 1 (IFN-γR1) expression, and can impact proteasome and/or aggresome degradation, endoplasmic reticulum function, cell cycle arrest, apoptosis, tumor microenvironment remodeling, and angiogenesis inhibition. In this study, we sought to elucidate the precise molecular mechanism by which Vorinostat inhibits HDACs. A deeper understanding of these mechanisms could improve our understanding of cancer cell abnormalities and provide new therapeutic possibilities for cancer treatment.
Keywords: Apoptosis; Cancer; Cell death; Clinical efficacy; Epigenetic pathways; Human and disease; Medicine; Oncology.
© 2024 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures

Similar articles
-
Molecular mechanisms underlying the clinical efficacy of panobinostat involve Stochasticity of epigenetic signaling, sensitization to anticancer drugs, and induction of cellular cell death related to cellular stresses.Biomed Pharmacother. 2023 Aug;164:114886. doi: 10.1016/j.biopha.2023.114886. Epub 2023 May 22. Biomed Pharmacother. 2023. PMID: 37224752
-
Vorinostat, a histone deacetylase (HDAC) inhibitor, promotes cell cycle arrest and re-sensitizes rituximab- and chemo-resistant lymphoma cells to chemotherapy agents.J Cancer Res Clin Oncol. 2016 Feb;142(2):379-87. doi: 10.1007/s00432-015-2026-y. Epub 2015 Aug 28. J Cancer Res Clin Oncol. 2016. PMID: 26314218
-
Histone deacetylase inhibitors selectively suppress expression of HDAC7.Mol Cancer Ther. 2007 Sep;6(9):2525-34. doi: 10.1158/1535-7163.MCT-07-0251. Mol Cancer Ther. 2007. PMID: 17876049
-
Anticancer clinical efficiency and stochastic mechanisms of belinostat.Biomed Pharmacother. 2023 Sep;165:115212. doi: 10.1016/j.biopha.2023.115212. Epub 2023 Aug 2. Biomed Pharmacother. 2023. PMID: 37541175 Review.
-
Regulation of apoptosis-associated genes by histone deacetylase inhibitors: implications in cancer therapy.Anticancer Drugs. 2010 Oct;21(9):805-13. doi: 10.1097/CAD.0b013e32833dad91. Anticancer Drugs. 2010. PMID: 20679890 Review.
References
-
- Hajam Y.A., Rani R., Ganie S.Y., Sheikh T.A., Javaid D., Qadri S.S., Pramodh S., Alsulimani A., Alkhanani M.F., Harakeh S., Hussain A., Haque S., Reshi M.S. Oxidative stress in human pathology and aging: molecular mechanisms and perspectives. Cells. 2022;11:552. doi: 10.3390/cells11030552. - DOI - PMC - PubMed
-
- Al-Griw M.A., Alshibani Z.O., Alghazeer R., Elhensheri M., Tabagh R.M., Eskandrani A.A., Alansari W.S., Habibulla M.M., Shamlan G. Histone deacetylase 2 inhibitor valproic acid attenuates bisphenol A-induced liver pathology in male mice. Sci. Rep. 2022;12 doi: 10.1038/s41598-022-12937-4. - DOI - PMC - PubMed
-
- Bouyahya A., Mechchate H., Oumeslakht L., Zeouk I., Aboulaghras S., Balahbib A., Zengin G., Kamal M.A., Gallo M., Montesano D. The role of epigenetic modifications in human cancers and the use of natural compounds as epidrugs: mechanistic pathways and pharmacodynamic actions. Biomolecules. 2022;12:367. - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

