Queen bee acid pretreatment attenuates myocardial ischemia/reperfusion injury by enhancing autophagic flux
- PMID: 39021954
- PMCID: PMC11253658
- DOI: 10.1016/j.heliyon.2024.e33371
Queen bee acid pretreatment attenuates myocardial ischemia/reperfusion injury by enhancing autophagic flux
Abstract
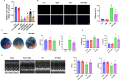
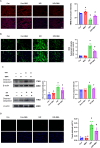
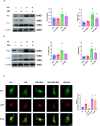
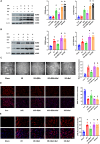
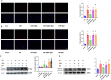
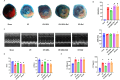
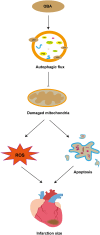
Queen bee acid (QBA), which is exclusively found in royal jelly, has anti-inflammatory, antihypercholesterolemic, and antiangiogenic effects. A recent study demonstrated that QBA enhances autophagic flux in the heart. Considering the significant role of autophagy in the development of myocardial ischemia/reperfusion (I/R) injury, we investigated the effect of pretreatment with QBA on myocardial damage. In an in vivo model, left coronary artery blockage for 30 min and reperfusion for 2 h were used to induce myocardial I/R. In an in vitro model, neonatal rat cardiomyocytes (NRCs) were exposed to 3 h of hypoxia and 3 h of reoxygenation (H/R). Our results showed that pretreatment with QBA increased the cell viability of cardiomyocytes exposed to H/R in a dose-dependent manner, and the best protective concentration of QBA was 100 μM. Next, we noted that QBA pretreatment (24h before H/R) enhanced autophagic flux and attenuated mitochondrial damage, cardiac oxidative stress and apoptosis in NRCs exposed to H/R injury, and these effects were weakened by cotreatment with the autophagy inhibitor bafilomycin A1 (Baf). In addition, similar results were observed when QBA (10 mg/kg) was injected intraperitoneally into I/R mice 30 min before ischemia. Compared to mice subjected to I/R alone, those treated with QBA had decreased myocardial infarct area and increased cardiac function, whereas, these effects were partly reversed by Baf. Notably, in NRCs exposed to H/R, tandem fluorescent mRFP-GFP-LC3 assays indicated increased autophagosome degradation due to the increase in autophagic flux upon QBA treatment, but coinjection of Baf blocked autophagic flux. In this investigation, no notable adverse effects of QBA were detected in either cellular or animal models. Our findings suggest that QBA pretreatment mitigates myocardial I/R injury by eliminating dysfunctional mitochondria and reducing reactive oxygen species via promoting autophagic flux.
Keywords: Apoptosis; Autophagic flux; Myocardial ischemia/reperfusion; Queen bee acid.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Gastrodin pretreatment alleviates myocardial ischemia/reperfusion injury through promoting autophagic flux.Biochem Biophys Res Commun. 2018 Sep 18;503(4):2421-2428. doi: 10.1016/j.bbrc.2018.06.171. Epub 2018 Jul 3. Biochem Biophys Res Commun. 2018. PMID: 29969626
-
Polydatin post-treatment alleviates myocardial ischaemia/reperfusion injury by promoting autophagic flux.Clin Sci (Lond). 2016 Sep 1;130(18):1641-53. doi: 10.1042/CS20160082. Epub 2016 Jun 23. Clin Sci (Lond). 2016. PMID: 27340138
-
Downregulation of LAPTM4B Contributes to the Impairment of the Autophagic Flux via Unopposed Activation of mTORC1 Signaling During Myocardial Ischemia/Reperfusion Injury.Circ Res. 2020 Sep 11;127(7):e148-e165. doi: 10.1161/CIRCRESAHA.119.316388. Epub 2020 Jul 22. Circ Res. 2020. PMID: 32693673
-
Trimetazidine Protects Cardiomyocytes Against Hypoxia/Reoxygenation Injury by Promoting AMP-activated Protein Kinase-dependent Autophagic Flux.J Cardiovasc Pharmacol. 2017 Jun;69(6):389-397. doi: 10.1097/FJC.0000000000000487. J Cardiovasc Pharmacol. 2017. PMID: 28581448
-
Postconditioning with Calreticulin Attenuates Myocardial Ischemia/Reperfusion Injury and Improves Autophagic Flux.Shock. 2020 Mar;53(3):363-372. doi: 10.1097/SHK.0000000000001387. Shock. 2020. PMID: 31135707
References
-
- Davidson S.M., et al. Multitarget Strategies to reduce myocardial ischemia/reperfusion injury. J. Am. Coll. Cardiol. 2019;73(1):89–99. - PubMed
-
- Bhatt D.L., Lopes R.D., Harrington R.A. Diagnosis and treatment of acute coronary syndromes: a review. JAMA. 2022;327(7):662–675. - PubMed
-
- Algoet M., et al. Myocardial ischemia-reperfusion injury and the influence of inflammation. Trends Cardiovasc. Med. 2023;33(6):357–366. - PubMed
-
- Heusch G. Myocardial ischaemia–reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020;17(12):773–789. - PubMed
LinkOut - more resources
Full Text Sources

