Identification of organs of origin of macrophages that produce presepsin via neutrophil extracellular trap phagocytosis
- PMID: 39013974
- PMCID: PMC11252129
- DOI: 10.1038/s41598-024-66916-y
Identification of organs of origin of macrophages that produce presepsin via neutrophil extracellular trap phagocytosis
Abstract
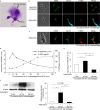
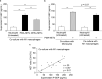
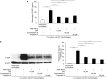
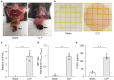
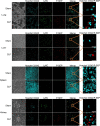
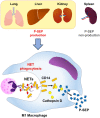
Presepsin (P-SEP) is a specific biomarker for sepsis. Monocytes produce P-SEP by phagocytosing neutrophil extracellular traps (NETs). Herein, we investigated whether M1 macrophages (M1 MΦs) are the primary producers of P-SEP after NET phagocytosis. We co-cultured M1 MΦs and NETs from healthy participants, measured P-SEP levels in the culture medium supernatant, and detected P-SEP using western blotting. When NETs were co-cultured with M1 MΦs, the P-SEP level of the culture supernatant was high. Notably, we demonstrated, for the first time, the intracellular kinetics of P-SEP production by M1 MΦs via NET phagocytosis: M1 MΦs produced P-SEP intracellularly 15 min after NET phagocytosis and then released it extracellularly. In a sepsis mouse model, the blood NET ratio and P-SEP levels, detected using ELISA, were significantly increased (p < 0.0001). Intracellular P-SEP analysis via flow cytometry demonstrated that lung, liver, and kidney MΦs produced large amounts of P-SEP. Therefore, we identified these organs as the origin of M1 MΦs that produce P-SEP during sepsis. Our data indicate that the P-SEP level reflects the trend of NETs, suggesting that monitoring P-SEP can be used to both assess NET-induced organ damage in the lungs, liver, and kidneys during sepsis and determine treatment efficacy.
Keywords: Biomarker; Macrophage; Neutrophil extracellular traps; Presepsin; Sepsis.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Presepsin production in monocyte/macrophage-mediated phagocytosis of neutrophil extracellular traps.Sci Rep. 2022 Apr 8;12(1):5978. doi: 10.1038/s41598-022-09926-y. Sci Rep. 2022. PMID: 35396366 Free PMC article.
-
Abnormal neutrophil traps and impaired efferocytosis contribute to liver injury and sepsis severity after binge alcohol use.J Hepatol. 2018 Nov;69(5):1145-1154. doi: 10.1016/j.jhep.2018.07.005. Epub 2018 Jul 18. J Hepatol. 2018. PMID: 30030149 Free PMC article.
-
Neutrophil Extracellular Trap Degradation by Differently Polarized Macrophage Subsets.Arterioscler Thromb Vasc Biol. 2020 Sep;40(9):2265-2278. doi: 10.1161/ATVBAHA.120.314883. Epub 2020 Jul 16. Arterioscler Thromb Vasc Biol. 2020. PMID: 32673525 Free PMC article.
-
The Impact of Cytokines on Neutrophils' Phagocytosis and NET Formation during Sepsis-A Review.Int J Mol Sci. 2022 May 3;23(9):5076. doi: 10.3390/ijms23095076. Int J Mol Sci. 2022. PMID: 35563475 Free PMC article. Review.
-
Neutrophil, neutrophil extracellular traps and endothelial cell dysfunction in sepsis.Clin Transl Med. 2023 Jan;13(1):e1170. doi: 10.1002/ctm2.1170. Clin Transl Med. 2023. PMID: 36629024 Free PMC article. Review.
Cited by
-
Presepsin Does Not Predict Risk of Death in Sepsis Patients Admitted to the Intensive Care Unit: A Prospective Single-Center Study.Biomedicines. 2024 Oct 11;12(10):2313. doi: 10.3390/biomedicines12102313. Biomedicines. 2024. PMID: 39457628 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

