Exploring treatment decision-making in chronic myeloid leukemia in chronic phase
- PMID: 39011484
- PMCID: PMC11246988
- DOI: 10.3389/fonc.2024.1369246
Exploring treatment decision-making in chronic myeloid leukemia in chronic phase
Abstract
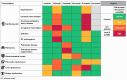
The introduction of tyrosine kinase inhibitors (TKIs) has transformed the treatment of chronic myeloid leukemia (CML). Each approved TKI has its own risk-benefit profile, and patients have choices across lines of therapy. Identifying the initial and subsequent treatment that will lead to the best possible outcome for individual patients is challenging. In this review, we summarize data for each approved TKI across lines of therapy in patients with CML in chronic phase, highlighting elements of each agent's safety and efficacy profile that may impact patient selection, and provide insights into individualized treatment sequencing decision-making aimed at optimizing patient outcomes.
Keywords: CML treatment; chronic phase; clinical decision-making; expert review; treatment selection; treatment sequencing.
Copyright © 2024 Andorsky, Kota and Sweet.
Conflict of interest statement
The authors declare the following potential conflicts of interest: DA received consulting fees and research funding from AbbVie and Celgene, and research funding from AstraZeneca and Novartis. KS received research funding from Jazz and Incyte and provides advisory board support/consulting for Gilead, Bristol Myers Squibb, Astellas, BerGenBio, Arog, Novartis, Curis, Pfizer, Mablytics, Daiichi Sankyo, Jazz, and Nkarta. VK received honorarium from Novartis and Pfizer. The authors declare that this study received funding from Novartis Pharmaceuticals Corporation. The funder had the following involvement in the study: provided financial support for medical editorial assistance.
Figures

Similar articles
-
Exploring Patient Decision Making Regarding Discontinuation of Tyrosine Kinase Inhibitors for Chronic Myeloid Leukemia.Oncologist. 2019 Sep;24(9):1253-1258. doi: 10.1634/theoncologist.2018-0831. Epub 2019 Apr 3. Oncologist. 2019. PMID: 30944185 Free PMC article.
-
Tyrosine Kinase Inhibitors in the Treatment of Chronic-Phase CML: Strategies for Frontline Decision-making.Curr Hematol Malig Rep. 2018 Jun;13(3):202-211. doi: 10.1007/s11899-018-0449-7. Curr Hematol Malig Rep. 2018. PMID: 29687320 Free PMC article. Review.
-
Use of tyrosine kinase inhibitors for chronic myeloid leukemia: management of patients and practical applications for pharmacy practitioners.Ann Pharmacother. 2011 Jun;45(6):787-97. doi: 10.1345/aph.1P784. Epub 2011 Jun 13. Ann Pharmacother. 2011. PMID: 21672900 Review.
-
Expert opinion-management of chronic myeloid leukemia after resistance to second-generation tyrosine kinase inhibitors.Leukemia. 2020 Jun;34(6):1495-1502. doi: 10.1038/s41375-020-0842-9. Epub 2020 May 4. Leukemia. 2020. PMID: 32366938 Free PMC article. Review.
-
Treating the chronic-phase chronic myeloid leukemia patient: which TKI, when to switch and when to stop?Expert Rev Hematol. 2017 Jul;10(7):659-674. doi: 10.1080/17474086.2017.1330144. Epub 2017 May 22. Expert Rev Hematol. 2017. PMID: 28511567 Review.
References
-
- Brümmendorf TH, Cortes JE, Milojkovic D, Gambacorti-Passerini C, Clark RE, le Coutre P, et al. . Bosutinib versus imatinib for newly diagnosed chronic phase chronic myeloid leukemia: final results from the BFORE trial. Leukemia. (2022) 36:1825–33. doi: 10.1038/s41375-022-01589-y - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

