Oncolytic vesicular stomatitis virus alone or in combination with JAK inhibitors is effective against ovarian cancer
- PMID: 39006945
- PMCID: PMC11246050
- DOI: 10.1016/j.omton.2024.200826
Oncolytic vesicular stomatitis virus alone or in combination with JAK inhibitors is effective against ovarian cancer
Abstract
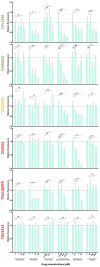
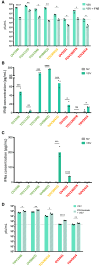
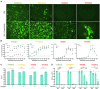
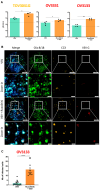
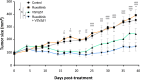
Therapy-resistant ovarian cancers have a poor prognosis and novel effective treatment options are urgently needed. In this study, we evaluated the therapeutic efficacy of the oncolytic vesicular stomatitis virus (VSV) against a panel of patient-derived ovarian cancer cell lines of all epithelial subtypes. Notably, we found that most of the cell lines were sensitive to VSV virotherapy. With the objective of improving treatment efficacy for the oncolytic virus-resistant cell lines, we tested various combinations with ovarian cancer standard of care drugs: olaparib, carboplatin, paclitaxel, doxorubicin, cyclophosphamide, and gemcitabine. While none of these combinations revealed to be beneficial, further experiments demonstrated that the antiviral interferon pathway was functional in VSV-resistant cell lines. Given that interferons signal through Janus kinase (JAK)-STAT to mediate their antiviral function, we tested combinations of oncolytic VSV with clinically relevant JAK inhibitors. Our results show that combining VSV with various JAK inhibitors, including ruxolitinib, enhances VSV virotherapy and treatment efficacy. Altogether, we show that VSV, either as a stand-alone treatment or in combination with JAK inhibitors provides an effective therapeutic option for ovarian cancer patients.
Keywords: JAK inhibitors; VSV; oncolytic virus; ovarian cancer; ruxolitinib.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
JAK/STAT inhibition with ruxolitinib enhances oncolytic virotherapy in non-small cell lung cancer models.Cancer Gene Ther. 2019 Nov;26(11-12):411-418. doi: 10.1038/s41417-018-0074-6. Epub 2019 Jan 9. Cancer Gene Ther. 2019. PMID: 30622322
-
Ruxolitinib and Polycation Combination Treatment Overcomes Multiple Mechanisms of Resistance of Pancreatic Cancer Cells to Oncolytic Vesicular Stomatitis Virus.J Virol. 2017 Jul 27;91(16):e00461-17. doi: 10.1128/JVI.00461-17. Print 2017 Aug 15. J Virol. 2017. PMID: 28566376 Free PMC article.
-
Overcoming cancer cell resistance to VSV oncolysis with JAK1/2 inhibitors.Cancer Gene Ther. 2013 Oct;20(10):582-9. doi: 10.1038/cgt.2013.55. Epub 2013 Sep 13. Cancer Gene Ther. 2013. PMID: 24030211 Free PMC article.
-
Expanding the Spectrum of Pancreatic Cancers Responsive to Vesicular Stomatitis Virus-Based Oncolytic Virotherapy: Challenges and Solutions.Cancers (Basel). 2021 Mar 9;13(5):1171. doi: 10.3390/cancers13051171. Cancers (Basel). 2021. PMID: 33803211 Free PMC article. Review.
-
VSV based virotherapy in ovarian cancer: the past, the present and …future?J Cancer. 2017 Jul 22;8(12):2369-2383. doi: 10.7150/jca.19473. eCollection 2017. J Cancer. 2017. PMID: 28819441 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
